Abstract
The Trömner sign is commonly used as a clinical neurological examination for upper motor neuron lesions above the fifth or sixth cervical segments of the spinal cord. This study aims to assess and quantify the Trömner signs utilizing electrophysiological test, and correlate to the severity of cord compression in cervical spondylotic myelopathy (CSM). We enlisted 46 CSM patients, and 30 healthy persons as controls. Manual Trömner and Hoffmann signs were tested in all subjects. By using a self-designed instrument, we performed electrophysiological assessments for the Trömner signs in patients and controls. Parameters of conduction latencies and amplitude of muscle action potentials were measured and compared with the cord compression ratios in CSM patients. The results showed a greater diagnostic sensitivity for the quantified Trömner signs in comparison to those of manual Trömner signs and Hoffmann signs. We found a positive correlation between the amplitude of muscle action potentials obtained in the Trömner signs and the cord compression ratios in the patients with CSM. In conclusion, the Trömner signs can be measured by electrophysiological assessments. We demonstrate a new quantification method for an established neurological sign. Not only is Trömner sign a highly sensitive test in clinical neurological examination, the electrophysiological assessment of this sign can also serve as an objective marker for evaluation of the severity of cervical cord compression.
Keywords: Trömner sign, Neurological reflex, Neurophysiology, Cervical spondylotic myelopathy
Introduction
The Trömner sign is flexion of the thumb and index finger in response to tapping or flicking the volar surface of the distal phalanx of the middle finger held partially flexed between the examiner’s finger and thumb [1]. It is an established neurological sign for pyramidal response in the upper extremity, and has been recognized as an alternative of Hoffmann’s sign [2]. The Hoffmann sign is usually elicited by nipping the nail or flicking the terminal phalanx of the middle or ring finger to elicit the finger flexor response. Both the Trömner and Hoffmann signs are commonly used as clinical neurological examinations for upper motor neuron lesions above the fifth or sixth cervical segments of the spinal cord.
Conventional studies on the Trömner signs described the presence or absence of response and showed the diversity in assessments in percentage of appearance. Otherwise, tests of this sign always require a practiced manipulation by the examiner, and the stimulation for eliciting this response cannot be standardized and quantified. In view of these, we hypothesize that this sign could be measured and quantified by electrophysiological assessments, and increase its diagnostic sensitivity in cervical cord compression disease. Therefore, the present study aims to evaluate and quantify the Trömner signs by an electrophysiological test, and compare its parameters to the severity of cord compression in patients with cervical spondylotic myelopathy (CSM).
Methods
Subjects
In this study, we enlisted 46 patients with clinical diagnosis of CSM. These patients included 29 men and 17 women with a mean age of 62 years (range 54–76). Thirty age-matched healthy persons recruited from patient spouses served as controls. Clinical diagnostic criteria for CSM included sensory impairments, muscular weakness, or associated hyper-reflexia in upper and lower extremities. Each patient had routine spinal roentgenography and magnetic resonance (MR) imaging examinations of the cervical spinal canal. The radiologic findings, accompanied by the clinical signs and features of cord compression, made the diagnosis of CSM. Durations of their disease from onset of myelopathy symptoms to electrophysiological evaluation ranged between 3 months and 2 years with a mean of 7.8 months. The neurological levels in these patients ranged from the fourth to the sixth cervical cords. The compression ratio of spinal cord was defined as the antero-posterior diameter divided by the transverse diameter of the cord measured in MR image at the compressed cord level. Measurement of the ratio of antero-posterior diameter to the transverse diameter of the cord was performed at the fifth cervical spinal levels in controls.
All CSM patients were recruited at random by the same physician from neurorehabilitation clinics. They were then referred to the neuro-physiological laboratory of a university teaching hospital for electrophysiological assessments of the Trömner signs during the period from July 2008 through December 2009. Manual tests of the Trömner and Hoffmann signs were performed before the electrophysiological tests. The electrophysiological test of the Hoffmann sign was not performed because of its inconsistency in quantification for nipping nail stimulation. By exclusion, no patient had any history of hand deformity, finger joint contracture, median nerve lesion, cervical radiculopathy, diabetes mellitus and uremia. We also excluded other causes of spinal cord lesions, such as trauma, infection, collagen diseases, or malignant disease with spinal metastasis. During the study, no medication, such as anti-spasmodic or anti-convulsive drugs was given.
The present study was approved by the university ethics committee in accordance with the international ethical standards of the 1964 Declaration of Helsinki. All patients accepted an informed consent before the study.
Quantification of the Trömner signs
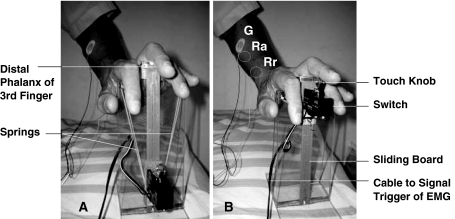
We assessed the patients and controls in sitting position and relaxed condition with the forearm prone position. In this way, we could minimize the actual motivational and emotional stress, as well as the influence of excitability in forearm flexors [3]. Upper extremities were selected at random side from the CSM patients and controls. We then put the subject’s hand and fingers gently on a self-designed instrument for examining the Trömner signs (Fig. 1a, b). To elicit the Trömner signs, the middle finger was stimulated on the volar surface of the distal phalanx with a knob attached on a switch. The switch was kept on a sliding board, and was connected to an EMG machine signal trigger (Synergy System, Medelec, Surrey, UK). When the holding springs were released, the switch was launched with a catapult mechanism and tapped the volar aspect of the distal middle finger phalanx. When performing the test for the Trömner signs, an estimate of skin area touched was 0.2 cm2, and the tap force was 1.2 N measured by a digital force indicator (CentorT Star, Com-Ten Industries, Florida, USA). We then placed the recording electrodes (Neuroline 710, Ambu Medicotest, Olsykke, Denmark) on the flexor digitorum superficialis muscle at forearm with a standard belly-tendon method, and placed the grounding electrode on the elbow proximal to the recordings. Each Trömner sign was tested five times. To minimize the habitual reaction of the reflex, we did the tests with an interval of 15 s between trials. Then, we recorded the shortest onset conduction latency as well as the maximum peak-to-peak amplitude of muscle action potentials (MAPs). An example of the test was shown in Fig. 2. During the electrophysiological tests, the skin temperature of the hand was kept constant above 30°C, with the assistance of an infrared lamp if necessary.
Fig. 1.
Instrument used for examining Trömner sign. a Before tapping. b tapping finger after release of springs, Ra active recording electrode, Rr reference recording electrode, G ground electrode
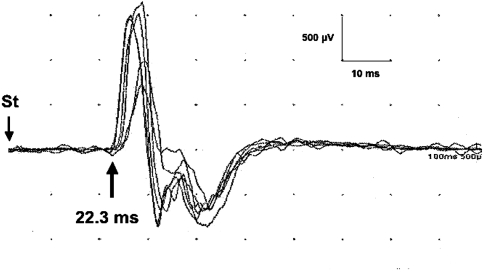
Fig. 2.
Example of the Trömner sign elicited in a patient with cervical spondylotic myelopathy. The shortest conduction latency was 22.3 ms and the maximum amplitude of muscle action potential was 2.5 mV. St stimulation
Statistical analyses
We performed statistical analyses for comparing the data between the patient groups and controls with chi-square test. The relationship between the MAP amplitude of the Trömner signs and the cord compression ratios in CSM patients was analyzed with Pearson correlation test. The maximal level of significance was 0.05. All statistical analyses were performed with SPSS for Windows (Version 15.0, SPSS Inc., Chicago, USA).
Results
Table 1 shows the test results of the Trömner and Hoffmann signs in CSM patients and controls. As can be seen, quantified electrophysiological tests showed positive Trömner signs in all CSM patients. However, manual Trömner signs were positive in 43 CSM patients, and their diagnostic sensitivities were 93.5%. The Hoffmann signs were positive in 41 patients, and their diagnostic sensitivities were 89.1%. No Trömner and Hoffmann signs appeared in controls.
Table 1.
Cord compression ratios and results of Trömner signs in CSM patients and controls
| Characteristics | CSM patients | Controls |
|---|---|---|
| No. of subjects | 46 | 30 |
| Cord compression ratios | 0.48 ± 0.12* | 0.77 ± 0.08* |
| Manual tests | ||
| Positive Trömner signs (diagnostic sensitivity) | 43 (93.5%) | 0 (NA) |
| Positive Hoffmann signs (diagnostic sensitivity) | 41 (89.1%) | 0 (NA) |
| Quantified tests | ||
| Positive Trömner signs (diagnostic sensitivity) | 46 (100%) | 0 (NA) |
| Conduction latency (ms) | 22.7 ± 3.4 | NA |
| Amplitude of MAP (mV) | 2.6 ± 0.8 | NA |
Values are mean ± standard deviation
No. number, MAP muscle action potential, NA not available
* χ2 = 0.832, p = 0.005, chi-square test
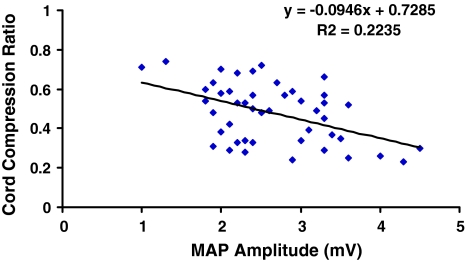
Cord compression ratios measured in CSM patients were 0.48 (SD = 0.12). This value was significantly smaller than the ratios of 0.77 (SD = 0.08) measured in the controls (χ2 = 0.832, p = 0.005). In comparison study of quantified Trömner signs and cord compression ratios, we found a negative linear relationship (Fig. 3) between the MAP amplitude of Trömner signs and the cord compression ratios in CSM patients (R2 = 0.2235, p = 0.017, Pearson correlation test). Patients with increased MAP amplitude of Trömner signs tend to have more serious spinal cord compression in CSM.
Fig. 3.
Correlation of cord compression ratios with the muscle action potential (MAP) amplitudes of Trömner signs in patients with cervical spondylotic myelopathy
Discussion
Physiologically, the Trömner sign is a stretch reflex response of the finger flexor, that is when a sensory input comes from the finger skin or tendon stretch, it may elicit a monosynaptic stretch reflex in Rexed lamina IX of the spinal cord [4] and coupling of muscles to other finger flexors [5]. This focal stretch response does not appear in normal adults because the excitabilities of spinal motoneurons are fully suppressed by the descending influence of supraspinal pathways. However, this response may activate and mediate through changes in spinal cord or cortico-spinal tract lesions of the spinal reflex circuits. According to the findings of Hiersemenzel [6] and Nielsen [7] in pathophysiology of spinal cord injury, adaptational changes in the excitability were found in the spinal neuronal circuits caudal to the spinal cord lesion. Great motoneurons are preliminary activated due to disturbed interaction of inhibitory and excitatory mechanisms on segmental levels. During the transition of spinal shock to spasticity, the reappearance of tendon reflex and muscle tone was associated with the recovery of spinal reflex and motor neuron excitability. This is the reason why the H/M (Hoffmann reflex/muscle action potential) ratio may reach its maximum in 8 weeks and remain stable thereafter [6]. Therefore, it was necessary to examine the Trömner sign in CSM patients, who were having spinal cord dysfunction at least 2 months after the onset of the disease.
Although the diagnostic sensitivity of the Trömner sign in cervical myelopathy and brain lesions has not yet been reported, we found that its sensitivity in CSM is higher than those of the Hoffmann signs in this study. This is probably due to the different stimulation applied onto the finger skin and nail. The Hoffmann sign is usually elicited by nipping the nail or flicking the terminal phalanx of the middle or ring finger, whereas the Trömner sign is elicited by tapping on the volar aspect of skin at the terminal phalanx of the middle finger. Anatomically, there are no nerve endings in the nail itself [8]. The nail acts as a counter force to the fingertip providing enhanced sensory input when an object is touched. If the manipulating stimulation intensity of nipping is inadequate to stimulate the nerve endings on the nail bed, it would lower the test sensitivity of the Hoffmann signs. On the other hand, direct stimulation on the skin over the volar aspect of the distal finger would be more sensitive than stimulation on the nail [9]. Therefore, the Trömner sign could show higher diagnostic sensitivity in comparison with the Hoffmann sign.
Compression ratio of spinal cord has been recognized as a strong indicator of spinal cord dysfunction [10] and served as a criterion for surgical treatment for CSM [11]. Present study shows a positive correlation between the MAP amplitude and the compression ratio of spinal cord. This finding indicates that increasing neuron excitabilities may be associated with increasing numbers of dis-inhibited motoneurons from central controls in the progression of spinal cord dysfunction in CSM. Thus, increase in activating neurons and their axonal fibers may lead to increase in MAP amplitude in the reflex responses. In spite of the findings in MAP amplitude, we found conduction latencies of Trömner signs were normal in CSM patients. This finding suggests that fastest nerve conductive fibers in the reflex arcs exist in CSM. Physiologically, different degrees of spinal cord lesions may affect different proportions of excited motoneurons below the cord lesion and change the MAP amplitude through the reflex responses. However, no peripheral nerve pathology occurred caudal to the spinal cord lesion in CSM. The short latency responses for stretch reflexes should be not affected [12] and their nerve conduction in the reflex arcs could still be intact.
In the literature, there exists little information on the ability of the Trömner sign to predict cervical cord compression. However, some previous reports have demonstrated that the Hoffmann sign, an alternative of the Trömner sign, was an early indication for diagnosis of CSM [13], and it was a sensitive marker for occult cervical cord compression [14]. In this study, we found the diagnostic sensitivity of the Trömner sign was higher than that of the Hoffmann sign in CSM patients. However, we did not investigate the specificity of the Trömner sign for the differential diagnosis of other diseases in the spinal cords, which merits further exploration.
In conclusion, the Trömner signs can be measured by electrophysiological assessments. In this study, we demonstrate a new quantification method for an established neurological sign. Not only is quantification of the Trömner sign a highly sensitive test in clinical neurological examination, the electrophysiological assessment for this sign can also serve as an objective marker for evaluation of the severity of cord compression in CSM.
Acknowledgments
The authors wish to thank Mr. Kai-Fong Chang for his technical assistance on the MR imaging of the spinal cords.
Conflict of interest None.
References
- 1.Larner AJ. A dictionary of neurological signs. 2. New York: Springer; 2006. p. 310. [Google Scholar]
- 2.Vogel P. The Trömner reflex: a containing misunderstanding? Nervenarzt. 1987;8:1–3. [PubMed] [Google Scholar]
- 3.Baldissera F, Bellani G, Cavallari P, Lalli S. Changes in the excitability of the H-reflex in wrist flexors related to the prone or supine position of the forearm in man. Neurosci Lett. 2000;295:105–108. doi: 10.1016/S0304-3940(00)01604-9. [DOI] [PubMed] [Google Scholar]
- 4.Harrop JS, Hanna A, Silva MT, Sharan A. Neurological manifestations of cervical spondylosis: an overview of signs, symptoms, and pathophysiology. Neurosurgery. 2007;60:S14–S20. doi: 10.1227/01.NEU.0000215380.71097.EC. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann G, Kamper DG, Kahn JH, Rymer WZ, Schmit BD. Modulation of stretch reflexes of the finger flexors by sensory feedback from the proximal upper limb post-stroke. J Neurophysiol. 2009;102:1420–1429. doi: 10.1152/jn.90950.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54:1574–1582. doi: 10.1212/wnl.54.8.1574. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity—from a basic science point of view. Acta Physiol (Oxf) 2007;189:171–180. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- 8.Champion RH, Burton JL, Ebling FJG. Textbook of dermatology. 5. Oxford: Blackwell; 1992. [Google Scholar]
- 9.Chang J, Vernadakis AJ, McClellan WT. Fingertip injuries. Clin Occup Environ Med. 2006;5:413–422. doi: 10.1016/j.coem.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Harrop JS, Naroji S, Maltenfort M et al (2010) Cervical myelopathy: a clinical and radiographic evaluation and correlation to cervical spondylotic myelopathy. Spine Feb. 10 (Epub ahead of print) [DOI] [PubMed]
- 11.Law MD, Jr, Bernhardt M, White AA., 3rd Cervical spondylotic myelopathy: a review of surgical indication and decision making. Yale J Biol Med. 1993;66:165–177. [PMC free article] [PubMed] [Google Scholar]
- 12.Toft E, Sinkjaer T, Espersen GT. Quantitation of the stretch reflex, technical procedures and clinical applications. Acta Neurol Scand. 1989;79:384–389. doi: 10.1111/j.1600-0404.1989.tb03805.x. [DOI] [PubMed] [Google Scholar]
- 13.Denno JJ, Meadows GR. Early diagnosis of cervical spondylotic myelopathy. Spine. 1991;16:1353–1355. doi: 10.1097/00007632-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Houten JK, Noce LA. Clinical correlations of cervical myelopathy and Hoffmann sign. J Neurosurg Spine. 2008;9:237–242. doi: 10.3171/SPI/2008/9/9/237. [DOI] [PubMed] [Google Scholar]