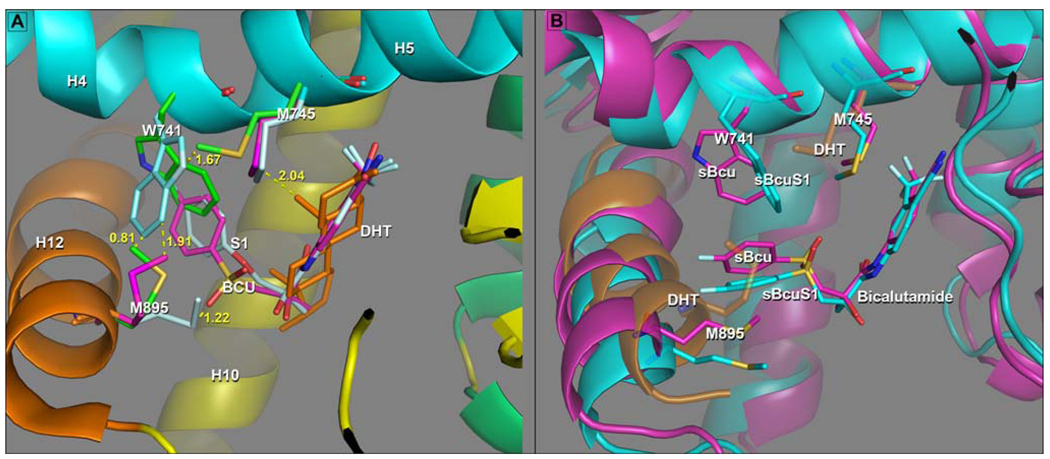
Figure 6.
Adaptation of AR LBP to the B-ring of Bcu and S1 in experimental and simulated complexes. In both panels A and B Helix 3 is not displayed to provide an unobstructed view of binding pocket interactions. A) When the sidechains can adapt to ligand in an agonist structure. Superposition of S1 (cyan), Bcu (magenta) and DHT (ligand gold: protein sidechains green, H12 gold, H4–H5 cyan, H10 yellow) experimental AR structures. This figure shows the shift of M895 from its position in the DHT complex (green carbons), away from H12 towards the ether oxygen of S1 (cyan) in order to accommodate the repositioned W741. This S1 configuration doesn’t accommodate Bcu in the agonist AR structure as when Bcu is bound, M895 in this position clashes with the sulfonyl group of Bcu. The orientation adopted by W741 in S1 to accommodate the B-ring brings it too close to the M745 side chain in DHT (green) and the configuration of M745 found in the S1 and Bcu complexes, which accommodates the rotated Trp741, is very close to the A-ring axial methyl group in DHT thus being disallowed in the DHT complex. B) When the sidechains are unable to adapt to ligand in agonist structure. Superposition of the 5ns structures for the sBcu simulation, colored magenta and the sBcuS1 simulation colored cyan. The semitransparent structure shows H12 and positions of M895 and M745 position in the reference AR-DHT complex (gold). This shows the re-positioning of the B-ring of Bcu “downward” and the concomitant displacement of H12 away from the LBS position, to create an enlarged pocket to accommodate the shifted B-ring. Repositioning of the B-ring is accomplished primarily by a rotation about the C11–C13 bond from ~ −60° to +60°, while the A-ring occupies the identical position it occupies in the W741L X-ray structure (Fig 4b). The displacement of H12 allows M895 to adopt an extended conformation back into the LBS without clashing with the sulfonyl group of Bcu or with the B-ring of Bcu as seen in the co-location of M895 in the DHT complex (gold) and the B-rings of Bcu. This shift of H12 occurs in both trajectories as does the positioning of M745 into a space similar to that it occupies in the S1 and W741L AR crystal structures.