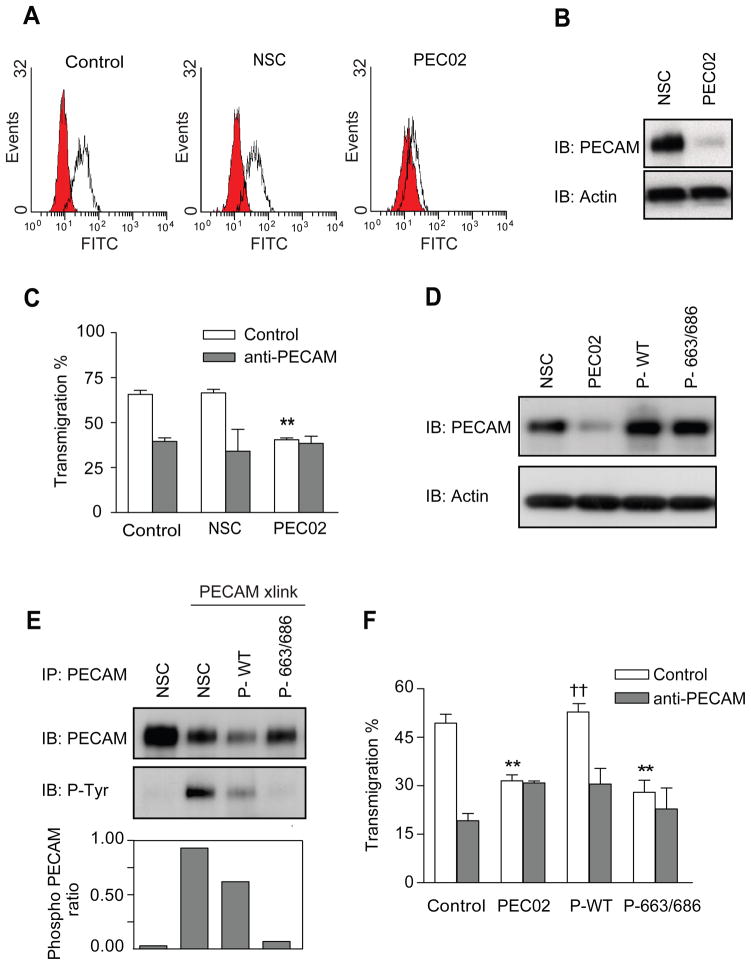
Figure 3. Tyrosine phosphorylation of leukocyte PECAM is required for transendothelial migration.
Control or stable U937L cells expressing either a control shRNA or an shRNA targeting PECAM (named NSC or PEC02 respectively) were analysed for PECAM expression by (A) flow cytometry and (B) western blotting. (C) Differentiated control and NSC U937L cells transmigrate across IL-1β treated HUVEC monolayers (200pg/ml, 4hours) to similar levels that are blocked in the presence of anti-PECAM antibodies. PEC02 cells show a reduced level of transmigration, which is not further inhibited by anti-PECAM antibodies. Data shown are mean ± SEM from 3 experiments, ** P<0.01. (D) Western blot analysis of lysates from NSC, PEC02 and PEC02 cells transduced to re-express either wild type (P-WT) or Y663/686F (P-663/686) forms of PECAM. Immuno-blotting was performed against PECAM and actin. (E) Mutating tyrosine sites 663 and 686 to phenylalanine reduces PECAM phosphorylation after crosslinking. Following PECAM cross-linking for 3 minutes, lysates from all the U937L cell lines were immunoprecipitated with anti-PECAM and probed for anti-phosphotyrosine. Ratios of phospho-PECAM in the cell lines are presented below the blots. (F) Differentiated U937L cell lines were allowed to transmigrate across IL-1β treated HUVEC monolayers for 1 hour, in the presence or absence of anti-PECAM antibodies. PEC02 cells re-expressing wild type (P-WT) PECAM, showed rescue of transmigration to levels similar to those on control U937L cells, †† P<0.02. Cells expressing the 663 and 686 mutant form of PECAM (P-663/686) remained inhibited in their transmigration, ** P<0.03, experiments show the mean ± SEM from 3 experiments.