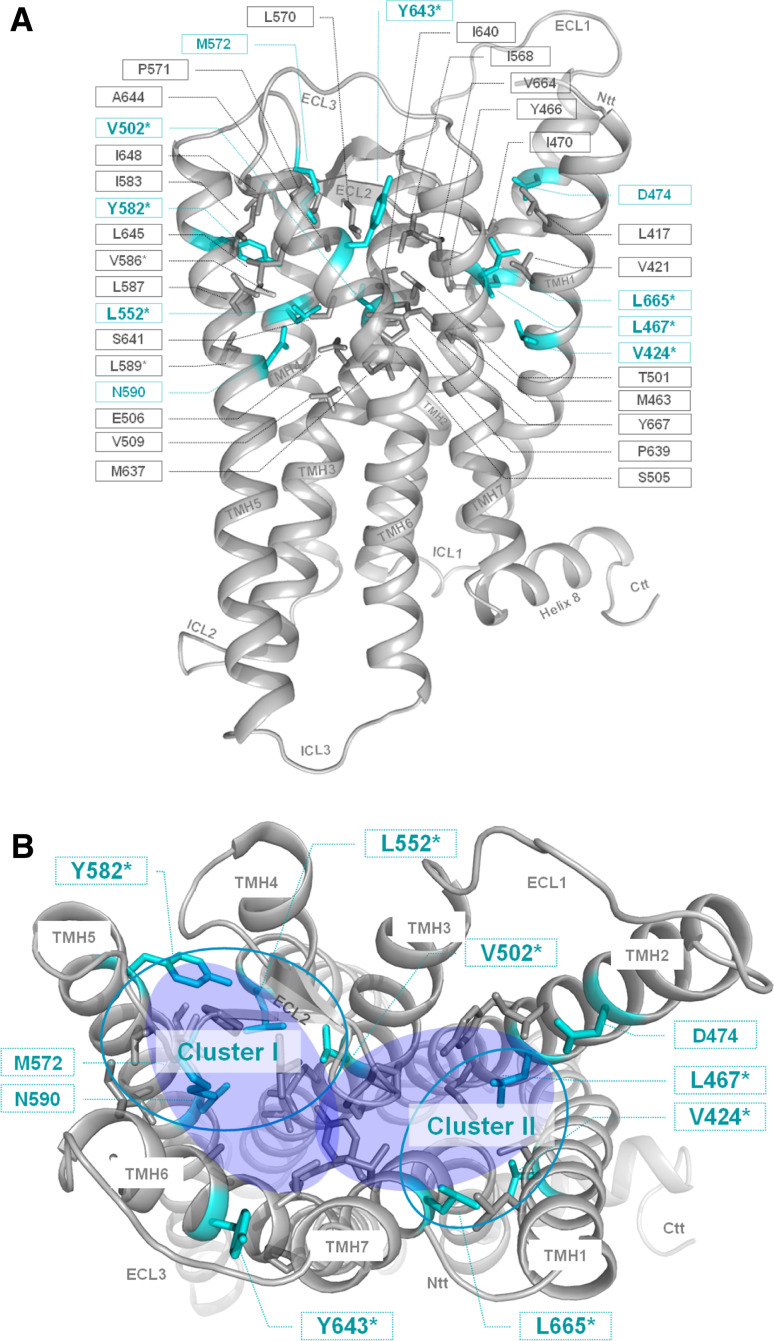
Fig. 3.
Amino acids of the potential allosteric binding pocket for small-molecule ligands in the thyrotropin receptor. The homology model of the transmembrane TSHR serpentine domain visualizes the spatial localization of 35 amino acids (sticks) that participate in the constitution of the transmembrane binding region for small drug-like molecules (TSHR specific numbering starting with the signal-peptide). Colored in cyan are residues whose mutations lead to an impaired signaling activity. Amino acids marked with the symbol asterisk were investigated in this study. For all other amino acids functional data from mutagenesis studies are published previously. a Side view (lateral to the membrane) of the transmembrane homology model. b In the top-view (from the extracellular side) only positions of inverse agonistic mutations are labeled and the potential allosteric binding region is highlighted with two translucent blue fully colored circles. The accumulation of inverse agonistic mutations (cyan circles) occurs between TMHs 3, 4, 5, ECL2 (Cluster I), and between TMHs 1, 2 and 7 (Cluster II)