Abstract
Study Objectives:
The prevalence of obstructive sleep apnea syndrome (OSAS) in sickle cell disease (SCD) has been reported to be higher than that in the general pediatric population. However, not all subjects with SCD develop OSAS. We hypothesized that SCD patients with OSAS have a blunted neuromuscular response to subatmospheric pressure loads during sleep, making them more likely to develop upper airway collapse.
Design:
Subjects with SCD with and without OSAS underwent pressure-flow measurements during sleep using intraoral surface electrodes to measure genioglossal EMG (EMGgg). Two techniques were applied to decrease the nasal pressure (PN) to subatmospheric levels, resulting in an activated and relatively hypotonic upper airway. The area under the curve of the inspiratory EMGgg moving time average was analyzed. EMGgg activity was expressed as a percentage of baseline. Changes in EMGgg in response to decrements in nasal pressure were expressed as the slope of the EMGgg vs. nasal pressure (slope of EMGgg-PN).
Setting:
Sleep laboratory.
Participants:
4 children with SCD and OSAS and 18 children with SCD but without OSAS.
Results:
The major findings of this study were: (1) using the activated but not the hypotonic technique, the slope of EMGgg-PN was more negative in SCD controls than SCD OSAS; (2) the slope of EMGgg-PN was significantly lower using the activated technique compared to the hypotonic technique in SCD controls only; (3) similarly, the critical closing pressure, Pcrit, was more negative using the activated technique than the hypotonic technique in SCD controls but not in SCD OSAS.
Conclusion:
This preliminary study has shown that children with SCD but without OSAS have more prominent upper airway reflexes than children with SCD and OSAS.
Citation:
Huang J; Pinto SJ; Allen JL; Arens R; Bowdre CY; Jawad AF; Mason TBA; Ohene-Frempong K; Smith-Whitely K; Marcus CL. Upper airway genioglossal activity in children with sickle cell disease. SLEEP 2011;34(6):773-778.
Keywords: Critical pressure, slow wave sleep, sleep disordered breathing
INTRODUCTION
The prevalence of the obstructive sleep apnea syndrome (OSAS) in patients with sickle cell disease (SCD) has been reported to be over 10%,1 which is manyfold higher than that among children in the general population.2 The pathophysiology of OSAS in children with SCD is not fully understood. Current knowledge of OSAS suggests that both anatomical and neuromuscular factors contribute to the pathogenesis of OSAS in children.3 Adenotonsillar hypertrophy is thought to be a major anatomical contributor to OSAS in patients with SCD. Children with SCD may be at increased risk for the development of adenotonsillar hypertrophy as a compensatory response to functional asplenia, or as a result of recurrent upper respiratory infections because of lack of opsonization of bacterial pathogens.4,5 However, even with enlarged tonsils and adenoids, children with SCD do not develop upper airway occlusions during wakefulness. Thus, it is possible that among those children with SCD and OSAS, the upper airway during sleep is more collapsible compared to children with SCD but no OSAS because of compromised upper airway neuromuscular activity, and hence obstructive events occur.
Normal upper airway neuromuscular responses depend on a functional central nervous system. However, cerebral vasculopathy is a major cause of morbidity and mortality in subjects with SCD. Cerebral vasculopathy can be manifested as overt strokes, which are cerebral infarctions with neurological symptoms, or silent infarcts, which are not associated with focal neurological symptoms.6 Ohene-Frempong et al. reported that 11% of patients with SCD had their first overt stroke by 20 years of age.7 Silent infarcts were detected with brain magnetic resonance imaging (MRI) in 22% of patients with SCD aged 6 to 19 years.8 In children with SCD younger than 6 years of age, the prevalence of silent infarct was reported to be similar.9 It is possible that cerebral vasculopathy in children with SCD results in abnormal regulation of upper airway muscles during sleep, and hence increases the risk of OSAS.
For this preliminary study, we hypothesized that SCD patients with OSAS have a blunted neuromuscular response to subatmospheric pressure loads during sleep, making them more likely to develop upper airway collapse.
METHODS
Subjects with SCD underwent baseline polysomnography using standard pediatric techniques.10 On a separate night, measurement of activated and hypotonic upper airway flow and genioglossal electromyographic (EMGgg) activity in response to subatmospheric upper airway pressure challenges during NREM sleep was conducted. Cerebral vasculopathy was evaluated using brain MRI. The Institutional Review Board at the Children's Hospital of Philadelphia approved the study. Informed consent was obtained from subjects aged 18 years and older, and from the parents or legal guardians of younger subjects. Assent was obtained from subjects aged 7 years and older.
Study Group
Subjects with SCD, aged 5-18 years, were recruited. The lower age limit was set to exclude those who were too young to cooperate with the face mask and other aspects of the protocol. Children who were treated with hydroxyurea, chronic transfusions, or previous adenotonsillectomy were excluded. Since children with SCD have lower baseline oxygen saturation,11 their oxygen saturation tends to be in the steep section of the oxygen-hemoglobin dissociation curve. Therefore, children with SCD tend to have more scorable obstructive hypopneas. For this study, subjects with an obstructive apnea hypopnea index (AHI) ≥ 3/h were recruited as cases of OSAS (SCD OSAS), and subjects with an AHI ≤ 1.5/h were recruited as controls (SCD control).12–15
Pressure-Flow and EMG Measurements
A dental impression of the lower anterior 4-5 teeth made of dental putty (Splash', Discus Dental, Inc., Culver City, CA) with surface stainless steel electrodes (A-M Systems, Inc., Sequim, WA) was customized for each subject.16–22 The subjects wore the dental mold throughout the sleep study. The surface electrodes were fitted to rest against the base of the genioglossal muscle to obtain upper EMG signal from the airway muscles, presumably primarily from the genioglossus.
Routine polysomnographic measurements were obtained. In addition, the subjects wore a continuous positive airway pressure (CPAP) full face mask (Philips Respironics, Murrysville, PA) attached to a heated pneumotachometer (Hans Rudolph, Inc., Kansas City, MO) and differential pressure transducer (ADInstruments, Colorado Springs, CO). Nasal pressure (PN) was measured at the mask, using a differential pressure transducer with a demodulator (Validyne Engineering Corp., Northridge, CA) referenced to atmosphere. Signals were acquired on a PowerLab system (ADInstruments, Colorado Springs, CO) and simultaneously displayed on a Rembrandt polysomnography system (Embla, Denver, CO). PN was altered in either a positive or subatmospheric direction, using a device provided by Philips Respironics.23,24 A toggle switch allowed the patient to be switched rapidly between positive and negative nasal pressure, ranging from −25 to +30 cm H2O.
Two techniques were applied to decrease the nasal pressure to subatmospheric (negative) levels, resulting in an activated and relatively hypotonic upper airway.19–21,25 Application of negative upper airway pressure results in activation of upper airway dilator muscles,26,27 whereas positive pressure suppresses EMGgg activity during sleep.28 Using the activated technique, the holding pressure, i.e., the highest PN at which flow limitation first became discernible, was determined. At that pressure level, upper airway muscle activities are relatively suppressed. The PN was then dropped in 2 cm H2O steps every 5 breaths to lower pressure levels until arousal or obstructive apnea occurred. This technique results in upper airway activation. Using the hypotonic technique, the studies were initiated at the holding pressure. PN was then acutely decreased by 2 cm H2O for 5 breaths, following which it was rapidly returned to the holding pressure. PN was dropped repeatedly to incrementally lower levels, with a return each time to the holding pressure, until either apnea or arousal occurred. Previous studies in children demonstrated that EMGgg is at a low level for the first 3 breaths of negative pressure, and begins to increase by the 4th breath.19–21 This technique results in an upper airway with minimal activation during the first 3 breaths of negative pressure. Because arousal often occurred with the trials, multiple trials using both the activated and the hypotonic technique were performed during NREM sleep. Trials performed during spontaneous respiratory events or other periods of respiratory instability were excluded from analysis. For each technique, the trial reaching the lowest PN before arousal/apnea was selected for data analysis.
Data Analysis
Pressure-flow curves were constructed based on analysis of flow-limited breaths, and the critical closing pressure (Pcrit) as well as the slope of the pressure-flow curve (SPF) were calculated.16,20,29 Pcrit was defined as the extrapolated nasal pressure at which flow reached zero. We used −25 cm H2O to represent Pcrit when the extrapolated Pcrit was < −25 cm H2O, since −25 cm H2O was the lowest pressure our device could provide.16,19,20,23 The SPF is the ratio of changes in flow to changes in nasal pressure, and is used to represent the conductance of the upper airway. A high SPF corresponds to an upper airway with low conductance.16 We used both Pcrit and SPF to characterize the flow response to changes in PN.
Raw EMGgg signals were rectified, and filtered at low frequency of 10 Hz and at high frequency of 100 Hz.10,17,18,21,30 The signals were then moving-time averaged with a time constant of 200 ms.21 The area under the curve of the inspiratory EMGgg moving-time average was calculated and corrected for inspiratory time (inspiratory EMGgg area under the curve/inspiratory time). The area under the curve of the EMGgg reflects total tonic and phasic inspiratory EMGgg activity. EMGgg activity was then presented as a percentage of baseline (the baseline being the averaged EMGgg activity over 5 breaths during inspiration at the holding PN prior to the pressure challenge). Changes in EMGgg in response to decrements in nasal pressure were presented as the slope of the EMGgg vs. nasal pressure (slope of EMGgg-PN).
Statistical Analysis
Statistical analysis was performed with SPSS software version 17.0 for windows (SPSS Inc., Chicago, IL). The Kolmogorov-Smirnov test was used to test for normalcy. Categorical data were compared using the χ2 test. Noncategorical data were presented as median and range, because most of the data were not normally distributed. Differences in Pcrit, SPF, and the slope of EMGgg-PN between activated and hypotonic techniques within the SCD OSAS or SCD control group were compared using the Wilcoxon signed-rank test. Differences in SCD OSAS compared to SCD controls were examined using the Mann-Whitney rank sum test. A P value < 0.05 was required for significance.
RESULTS
Four SCD OSAS and 18 SCD controls were studied. Subject characteristics are shown in Table 1. As planned, SCD OSAS had a significantly higher AHI than SCD controls (P < 0.001). Three SCD controls but no SCD OSAS had cerebral vasculopathy diagnosed with brain MRI.
Table 1.
Demographic and polysomnographic data
| SCD OSAS (n = 4) | SCD Controls (n = 18) | |
|---|---|---|
| Age (years) | 15 (13 to 17) | 14 (7 to 18) |
| Male, (N, %) | 3 (75) | 9 (50) |
| Body mass index Z-score | −0.5 (−3.3 to −0.4) | −0.4 (−3.1 to 1.0) |
| Apnea hypopnea index (N/h) | 6.7 (3.0 to 27.0) | 0.3 (0.0 to 1.1)* |
| Arterial oxygen saturation nadir (%) | 80 (69 to 91) | 87 (71 to 96) |
| Peak end-tidal CO2 (mm Hg) | 53 (47 to 58) | 53 (41 to 61) |
| Cerebral vasculopathy (N) | 0 | 3 |
P < 0.001. Data presented as median (range) unless otherwise specified.
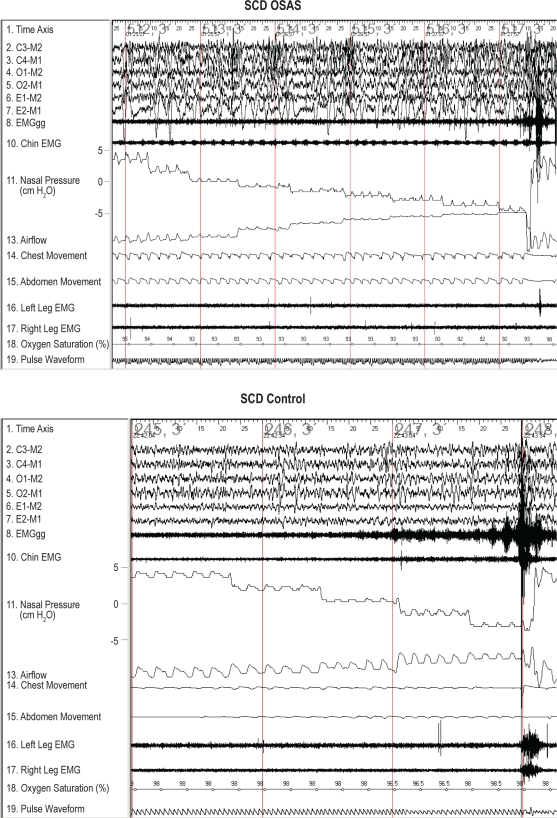
Typical examples of pressure-flow runs are shown in Figure 1.
Figure 1.
Typical examples of airflow and EMGgg changes during pressure-flow runs in slow wave sleep are shown for a subject with SCD and OSAS and an SCD control subject. During inspiration, airflow and nasal pressure signal tracings have a negative deflection. Initially, nasal pressure was maintained at the holding pressure. The nasal pressure was then lowered in 2 cm H2O steplike decrements every 5 breaths until arousal or obstructive apnea occurred. The baseline of the airflow tracings for both subjects moved upwards during the pressure drops due to the change in bias flow. The subject with SCD OSAS had a decrease in airflow with no significant EMGgg increase in response to negative pressure, whereas the SCD control maintained airflow and had a gradually increased EMGgg.
Slope of EMGgg-PN (Table 2 and Figure 2)
Table 2.
Slopes of pressure-flow curve, critical closing pressure and slopes of EMGgg-PN from sickle cell disease subjects with and without OSAS using activated or hypotonic technique
| SCD OSAS |
SCD Controls |
|||
|---|---|---|---|---|
| Activated | Hypotonic | Activated | Hypotonic | |
| Slopes of EMGgg-PN (%/cm H2O) | ||||
| Median | −3.3a | −3.8 | −11.6 | −1.9b |
| Range | −7.1 to 3.5 | −4.0 to 3.3 | −27.9 to −1.9 | −11.3 to 1.5 |
| 95% CI | −9.6 to 4.5 | −7.8 to 3.6 | −17.8 to −8.0 | −5.9 to −1.6 |
| Pcrit (cm H2O) | ||||
| Median | −17.4 | −14.5 | −25.0 | −25.0c |
| Range | −25.0 to −4.2 | −25.0 to −3.7 | −25.0 to −6.3 | −25.0 to −4.2 |
| 95% CI | −32.9 to 0.9 | −33.9 to −5.0 | −25.0 to −19.0 | −21.6 to −14.8 |
| Slopes of pressure-flow curve (mL/sec*cm H2O) | ||||
| Median | 10.6 | 25.7 | 4.5 | 10.8 |
| Range | −5.1 to 60.3 | 1.0 to 72.7 | −10.3 to 37.4 | −21.2 to 32.4 |
| 95% CI | −27.1 to 65.3 | −24.5 to 87.1 | 1.4 to 14.8 | 3.7 to 18.0 |
| Holding pressure (cm H2O) | ||||
| Median | 3.1 | 3.3 | 1.2 | 1.5 |
| Range | 1.3 to 7.0 | 1.3 to 7.8 | −1.8 to 5.1 | −1.0 to 5.0 |
| 95% CI | −0.8 to 8.0 | −1.0 to 8.8 | 0.4 to 2.4 | 0.9 to 2.5 |
| Lowest pressure before arousal/apnea (cm H2O) | ||||
| Median | −6.3d | −3.4e | −13.0 | −13.0 |
| Range | −6.8 to −4.4 | −15.0 to −3.3 | −20.3 to −3.1 | −23.6 to −5.2 |
| 95% CI | −7.7 to −4.2 | −15.6 to 3.4 | −19.2 to −9.8 | −10.4 to −15.1 |
SCD controls had more negative slopes of EMGgg-PN than SCD OSAS when using the activated technique (P = 0.04).
For SCD controls, the slope of EMGgg-PN using the activated technique was more negative than that using the hypotonic technique (P < 0.001).
For SCD controls, Pcrit was more negative using the activated technique than the hypotonic technique (P = 0.03).
SCD controls had a more negative lowest pressure before arousal/apnea than SCD OSAS using the activated technique (P = 0.03).
SCD controls had a more negative lowest pressure before arousal/apnea than SCD OSAS using the hypotonic technique (P = 0.02).
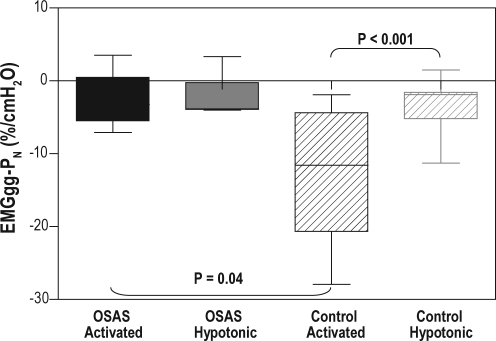
Figure 2.
Box and whiskers plot of the slopes of the EMGgg-PN data SCD OSAS and SCD controls. The boxes represent the interquartile range that contains 50% of the values. The line across each box indicates the median. The whiskers extend from the box to the highest and lowest values. Solid boxes represent data obtained from the SCD OSAS group; striped boxes represent data obtained from the SCD control group. Black boxes represent data obtained using the activated technique; gray boxes represent data obtained using the hypotonic technique. The slope of the EMGgg-PN (as a percentage of the baseline EMGgg) using the activated technique was more negative in SCD controls than in SCD OSAS (P = 0.04), but the slope of EMGgg-PN using the hypotonic technique was similar between the groups. In SCD controls but not in SCD OSAS, the slope of the EMGgg-PN was more negative using the activated technique than using the hypotonic technique (P < 0.001).
For SCD OSAS, no difference in the slope of EMGgg-PN was observed using activated or hypotonic techniques. For SCD controls, the slope was significantly more negative using the activated technique compared to the hypotonic technique (P < 0.001), reflecting increased genioglossal activity. The slope of EMGgg-PN using the activated technique was more negative in SCD controls than in SCD OSAS (P = 0.04), indicating that upper airway muscle activation was more prominent in SCD controls than in SCD OSAS. EMGgg-PN using the hypotonic technique was similar between groups.
Critical Closing Pressure (Table 2 and Figure 3)
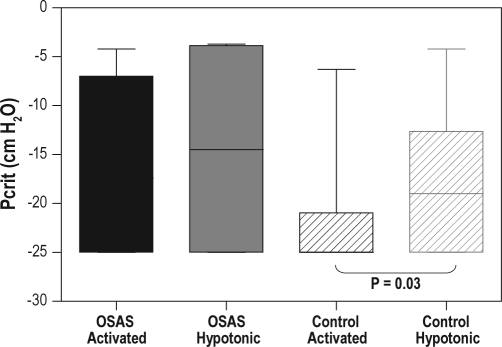
Figure 3.
Box and whiskers plot of the critical closing pressure (Pcrit) data for SCD OSAS and SCD controls. The boxes represent the interquartile range that contains 50% of the values. The lines across the boxes indicate the median. The whiskers extend from the boxes to the highest and lowest values. Solid boxes represent data obtained from the SCD OSAS group; striped boxes represent data obtained from the SCD control group. Black boxes represent data obtained using the activated technique; gray boxes represent data obtained using the hypotonic technique. For SCD controls, Pcrit obtained using the activated technique was significantly lower than that using the hypotonic technique (P = 0.03). Note that a floor value of −25 cm H2O was used.
Group Pcrit data are shown in Table 2. The activated Pcrit values from the 4 SCD OSAS subjects were −4.2, −9.8, −25, and −25 cm H2O, and the hypotonic Pcrit values from the same subjects were −3.7, −4.0, −25, and −25 cm H2O, respectively. For SCD OSAS, no difference in Pcrit was observed between the activated and hypotonic techniques. However, for SCD controls, Pcrit obtained using the activated technique was significantly lower (i.e., more negative) than that using the hypotonic technique (P = 0.03), reflecting a significant contribution of upper airway muscle activation to upper airway patency. There was a trend for a higher (more positive) Pcrit under both activated and hypotonic conditions for SCD OSAS compared to SCD controls. The difference in Pcrit between the 2 groups did not reach significance, probably due to the small number of subjects with SCD and OSAS. The difference between the activated and hypotonic Pcrit (ΔPcrit) in each group was calculated. The ΔPcrit values from the 4 SCD OSAS subjects were −6.1, −0.2, 0, and 0 cm H2O, respectively. No difference in ΔPcrit was observed between the 2 groups. Existence of a negative ΔPcrit suggests the presence of active neuromotor reflexes during sleep. Among the SCD OSAS group, only one subject (25%) had a negative ΔPcrit, whereas among the SCD control group, ΔPcrit was negative in 8 subjects (44%, P = NS).
Slope of Pressure-Flow Curve (Table 2)
The activated SPF values from the 4 SCD OSAS subjects were −5.1, 3.7, 17.5, and 60.3 mL/sec*cm H2O, and the hypotonic SPF values from the same subjects were 1.0, 3.4, 48, and 73 mL/sec*cm H2O, respectively. Within the SCD OSAS and SCD control groups, no differences in SPF were observed using the activated or hypotonic technique. Similar to Pcrit, a trend for a steeper SPF using both techniques for SCD OSAS vs. SCD controls was observed.
Data on the holding pressure and the lowest pressure before arousal/apnea were compared. No difference in holding pressure was observed between the SCD OSAS and SCD control groups.
Data on slope of EMGgg-PN, Pcrit, slope of pressure-flow curve, holding pressure and lowest pressure before arousal/apnea were compared between the 3 SCD controls with cerebral vasculopathy and the rest of the SCD controls. No significant differences in the above parameters were observed between the 2 subgroups.
DISCUSSION
This is a pilot study with a small number of subjects. The major findings of this study were: (1) using the activated but not the hypotonic technique, the slope of EMGgg-PN was more negative in SCD controls than SCD OSAS; (2) the slope of EMGgg-PN and Pcrit using the hypotonic technique were similar between the two groups; (3) the slope of EMGgg-PN was significantly more negative using the activated technique compared to the hypotonic technique in SCD controls only; (4) similarly, Pcrit was more negative using the activated technique than the hypotonic technique in SCD controls but not in SCD OSAS. Overall, we thus demonstrated that upper airway reflexes were more prominent in SCD controls than in SCD OSAS, indicating that decreased upper airway neuromuscular reflexes contribute to the increased upper airway collapsibility in children with SCD and OSAS.
Compared to the hypotonic technique, the activated technique results in a more activated upper airway (more negative Pcrit and steeper slope of EMGgg-PN) in normal children.19–21 Differences in Pcrit using the two techniques in SCD control demonstrated the contribution of upper airway reflexes to upper airway patency during sleep. However, those reflexes were blunted in SCD OSAS. It is possible that covert cerebral vasculopathy in some children with SCD causes impairment of active upper airway reflexes during sleep, so that they are unable to maintain upper airway patency. Although none of the subjects with SCD OSAS had imaging proof of cerebral vasculopathy, it is still possible that these subjects had subclinical neurologic damage secondary to vaso-occlusive episodes. Changes in upper airway local reflexes, abnormal central processing of the afferent stimuli, and abnormal control of upper airway dilators may contribute to the impairment of upper airway responses to subatmospheric pressure, and need to be further explored. More detailed EEG analysis, such as frequency-specific EEG power analysis31 or respiratory-related evoked potentials,32 or more sophisticated imaging techniques may provide further insight into the pathophysiology of OSAS in SCD.
Only a small number of children with SCD and OSAS were recruited. This is because our technique cannot be used easily in very young children, in whom OSAS is more common. Also, children undergoing treatment with hydroxyurea were excluded, and at our institution hydroxyurea has become a standard treatment for children with significant complications of SCD. Nevertheless, even with this small number of cases, we were able to demonstrate a significant difference in EMG reflexes between children with SCD with and without OSAS.
Previous studies have typically shown a higher Pcrit in children with OSAS than that found in the SCD OSAS subjects in the current study. However, this may be due to the variability typically shown in these measurements; data for Pcrit and the slope of the pressure-flow relationship are within the range previously reported.20 In addition, as all the SCD OSAS subjects were underweight, they may have had a lower anatomical load on the upper airway compared to children with OSAS but no SCD. In addition, it would be beneficial to have data on the size of the tonsils and adenoids to further evaluate the contribution of anatomic factors to upper airway patency in children with OSAS and SCD. This should be a direction for future research.
It would have been ideal to have included a control group of subjects with OSAS but no SCD. However, most subjects in the study were adolescents, and OSAS is rare in otherwise normal adolescents without obesity. In contrast, children with SCD are underweight.33 Previous data have demonstrated that obesity increases upper airway collapsibility.34 Therefore in this study, we did not include children with OSAS but no SCD.
To conclude, this preliminary study has shown that children with SCD but without OSAS have more prominent upper airway reflexes than children with SCD and OSAS. We speculate that decreased upper airway neuromuscular reflexes contribute to the increased upper airway collapsibility in children with SCD and OSAS.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Marcus has received research support and equipment from Phillips Respironics unrelated to this study. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by NIH grants UL1 RR024134, R01 HL79911 and R01 HL58585. Philips Respironics, Inc. provided the airway pressure device. The authors thank all of the Children's Hospital of Philadelphia sleep laboratory technologists who helped conduct this study. We are grateful to the children and their families for their enthusiastic participation in this study.
REFERENCES
- 1.Salles C, Ramos RT, Daltro C, Barral A, Marinho JM, Matos MA. Prevalence of obstructive sleep apnea in children and adolescents with sickle cell anemia. J Bras Pneumol. 2009;35:1075–83. doi: 10.1590/s1806-37132009001100004. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 3.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 4.Maddern BR, Reed HT, Ohene-Frempong K, Beckerman RC. Obstructive sleep apnea syndrome in sickle cell disease. Ann Otol Rhinol Laryngol. 1989;98:174–8. doi: 10.1177/000348948909800302. [DOI] [PubMed] [Google Scholar]
- 5.Samuels MP, Stebbens VA, Davies SC, Picton-Jones E, Southall DP. Sleep related upper airway obstruction and hypoxaemia in sickle cell disease. Arch Dis Child. 1992;67:925–9. doi: 10.1136/adc.67.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006;5:501–12. doi: 10.1016/S1474-4422(06)70469-0. [DOI] [PubMed] [Google Scholar]
- 7.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–94. [PubMed] [Google Scholar]
- 8.Pegelow CH, Macklin EA, Moser FG, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood. 2002;99:3014–8. doi: 10.1182/blood.v99.8.3014. [DOI] [PubMed] [Google Scholar]
- 9.Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009;146:300–5. doi: 10.1111/j.1365-2141.2009.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 11.Needleman JP, Setty BN, Varlotta L, Dampier C, Allen JL. Measurement of hemoglobin saturation by oxygen in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:423–8. doi: 10.1002/(sici)1099-0496(199912)28:6<423::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 13.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 14.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 15.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 16.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol. 1999;87:626–33. doi: 10.1152/jappl.1999.87.2.626. [DOI] [PubMed] [Google Scholar]
- 17.Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am J Respir Crit Care Med. 2003;168:664–70. doi: 10.1164/rccm.200301-092OC. [DOI] [PubMed] [Google Scholar]
- 18.Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–60. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- 19.Marcus CL, Fernandes Do Prado LB, Lutz J, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 20.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005;57:99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 21.Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. 2006;173:902–9. doi: 10.1164/rccm.200509-1450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Pinto SJ, Katz ES, et al. Genioglossus activity in obese, non-snoring adolescents during sleep. Sleep. 2009;32:A169. [Google Scholar]
- 23.Bandla P, Huang J, Karamessinis L, et al. Puberty and upper airway dynamics during sleep. Sleep. 2008;31:534–41. doi: 10.1093/sleep/31.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Karamessinis LR, Pepe ME, et al. Upper airway collapsibility during REM sleep in children with the obstructive sleep apnea syndrome. Sleep. 2009;32:1173–81. doi: 10.1093/sleep/32.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz AR, O'Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea - Role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998;157:1051–7. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 26.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horner RL, Innes JA, Morrell MJ, Shea SA, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol. 1994;476:141–51. [PMC free article] [PubMed] [Google Scholar]
- 28.Strohl KP, Redline S. Nasal CPAP therapy, upper airway muscle activation, and obstructive sleep apnea. Am Rev Respir Dis. 1986;134:555–8. doi: 10.1164/arrd.1986.134.3.555. [DOI] [PubMed] [Google Scholar]
- 29.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77:918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 30.Redfern MS, Hughes RE, Chaffin DB. High-pass filtering to remove electrocardiographic interference from torso EMG recordings. Clin Biomech (Bristol, Avon) 1993;8:44–8. doi: 10.1016/S0268-0033(05)80009-9. [DOI] [PubMed] [Google Scholar]
- 31.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Method for detection of respiratory cycle-related EEG changes in sleep-disordered breathing. Sleep. 2004;27:110–5. doi: 10.1093/sleep/27.1.110. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Colrain IM, Melendres MC, et al. Cortical processing of respiratory afferent stimuli during sleep in children with the obstructive sleep apnea syndrome. Sleep. 2008;31:403–10. doi: 10.1093/sleep/31.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell MJ, Carpenter GJO, Crosby LE, Bishop CT, Hines J, Noll J. Growth status in children and adolescents with sickle cell disease. Pediatr Hematol Oncol. 2009;26:202–15. doi: 10.1080/08880010902896882. [DOI] [PubMed] [Google Scholar]
- 34.Kirkness JP, Schwartz AR, Schneider H, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol. 2008;104:1618–24. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]