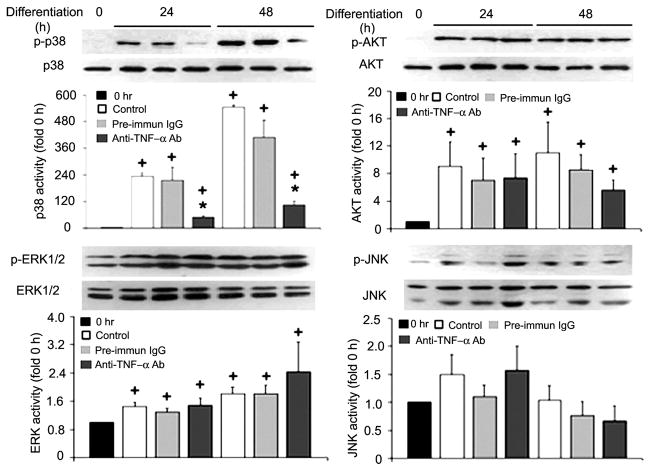
Fig. 2.
Myoblast-released TNF-α is critical to p38 activation in differentiating C2C12 myoblasts. Differentiation of C2C12 myoblasts was induced by replacing the GM with DM. TNF-α-neutralizing antibody (5 μg/ml) or preimmune IgG (pre-immun IgG; 5 μg/ml) was included in the DM, as indicated. At the indicated times, myoblasts were collected and processed for Western blot analysis with antibodies (Ab) against phosphorylated or pan-p38, Akt, ERK1/2, and JNK. Representative blots are shown for each of the kinases and were derived from 3 independent experiments. Kinase activation, normalized against the relevant total protein, was quantified by measuring the optical density of phosphorylated kinases on the X-ray film. Data are expressed as x-fold of the level at 0 h. Means ± SE (n = 3) were analyzed via ANOVA (P < 0.05), followed by the Fisher’s LSD multiple-comparison test. +Difference from 0 h (P < 0.05). *Difference from the control within the same time groups (24 or 48 h) (P < 0.05).