Abstract
Activation of the aryl hydrocarbon receptor (AHR), an environment-sensing transcription factor, causes profound impairment of mammary gland differentiation during pregnancy. Defects include decreased ductal branching, poorly formed alveolar structures, suppressed expression of milk proteins, and failure to nutritionally support offspring. AHR is activated by numerous environmental toxins, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and plays an as yet poorly understood role in development and reproduction. To better understand how AHR activation affects pregnancy-associated mammary gland differentiation, we used a combination of ex vivo differentiation, mammary epithelial transplantation, and AHR-deficient mice to determine whether AHR modulates mammary development through a direct effect on mammary epithelial cells (MECs) or by altering paracrine or systemic factors that drive pregnancy-associated differentiation. Studies using mutant mice that express an AHR protein lacking the DNA-binding domain show that defects in pregnancy-associated differentiation require AHR:DNA interactions. We then used fluorescence-based cell sorting to compare changes in gene expression in MECs and whole mammary tissue to gain insight into affected signaling pathways. Our data indicate that activation of the AHR during pregnancy directly affects mammary tissue development via both a direct effect on MECs and through changes in cells of the fat pad, and point to gene targets in MECs and stromal tissues as putative AHR targets.
Keywords: agalactia, alveolarization, environment, environmental factors, lactation, lactogenesis, mammary glands, TCDD, toxicology
Aryl hydrocarbon receptor targets in mammary epithelium and fat pad contribute to impaired mammary gland development during pregnancy.
INTRODUCTION
The mammary gland develops mostly during postnatal life. At birth, it is composed of a simple network of ducts and terminal end buds (TEBs), which are highly proliferative immature structures. With the onset of puberty, under the control of reproductive hormones, the TEBs proliferate and differentiate, and secondary and tertiary ducts invade the fat pad, after which glandular development is minimal [1]. During pregnancy, the gland resumes its growth, with a highly proliferative phase in the beginning of pregnancy, when ductal branching takes place, followed by a phase in which epithelial cells differentiate into lobule-alveolar structures that will eventually produce milk [2, 3]. This complex process of postnatal mammary development is coordinated by numerous molecules that are produced both systemically, in several glands and tissues, and locally by the cells of the fat pad (stroma) and mammary epithelium [4–6]. Extrinsic factors such as nutrition and anthropogenic chemicals affect and potentially deregulate this well-orchestrated process [7–12]. One target pathway for exogenous chemicals to influence mammary gland development is via activation of the aryl hydrocarbon receptor (AHR). Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which is an AHR-specific agonist and common environmental pollutant, causes severe defects in mammary gland development [12]. During pregnancy, injuries to the gland include decreased epithelial cell proliferation in early pregnancy and midpregnancy, stunted longitudinal growth, decreased ductal branching, poor formation of alveolar structures, and reduced expression of milk proteins. Collectively, these defects in development result in an inability to nutritionally support offspring [9, 12].
The AHR is a member of the per-arnt-sim (PAS) family of basic-region-helix-loop-helix (bHLH) transcription factors and directs the expression of many detoxification genes [13]. Upon activation by ligands such as TCDD, the AHR translocates to the nucleus and induces the expression of genes for several metabolic enzymes. Thus, one role of AHR is to provide protection from toxic chemicals by stimulating expression of enzymes to eliminate them. In addition to promoting metabolism and clearance of xenobiotics, recent studies suggest that AHR modulates physiological processes and molecular cascades that regulate proliferation, differentiation, and apoptosis [14–17]. Moreover, phenotypic differences such as decreased liver development, decreased body size over the first 4 wk of age, altered heart, ovary, and other organ development, and increased mortality rate were noted in AHR knockout (AhrKO) mice when compared to wild-type (WT) littermates [18–21], further emphasizing that there is a normal, physiological role for AHR.
Although it has not been thoroughly evaluated, data regarding the AHR's role in mammary gland development present a mixed picture, but collectively they suggest a possible endogenous role of the AHR in pregnancy-associated mammary development [22–24]. Moreover it is clear that AHR activation by exogenous ligands causes severe defects in pregnancy-associated mammary gland growth and lactogenesis [9, 12]. Understanding the precise molecular mechanisms that control mammary gland development during pregnancy remains an active area of research. Work from numerous laboratories has shown that specific factors regulate distinct phases of this process; thus, there are many potential molecular targets of AHR [4, 25–27]. We have previously reported that TCDD does not cause defects in lactogenic differentiation by simply altering plasma levels of prolactin, estradiol, or progesterone [12]. However, in addition to circulating hormone levels, development of the mammary gland is controlled by many other molecules that are produced locally by the cells of the mammary fat pad and epithelium, and systemically by other organs, such as the liver [2, 4, 26, 27]. Failed lactogenesis could therefore result from AHR-mediated direct effects on mammary gland tissues or indirectly by disrupting levels of systemically derived regulatory factors. Furthermore, within the mammary gland itself, molecules derived from the stroma (e.g., fat pad) and parenchymal tissues (e.g., mammary epithelial cells) regulate the process of lactogenesis, and TCDD exposure could perturb events within either or both of these compartments.
The objectives of the present study were to determine whether: 1) the effects of TCDD on pregnancy-associated mammary development are mediated by the AHR and if the loss of AHR's DNA-binding domain alters the normal pregnancy-associated mammary development, (2) exposure to TCDD during pregnancy alters mammary development through a direct effect on the mammary tissue, and (3) defects in mammary differentiation and lactogenesis result from direct effects on mammary epithelial cells (MECs). Determining whether AHR activation by TCDD affects the normal course of mammary development by altering processes that occur locally in the mammary gland and within the fat pad and/or the epithelium is critical for identifying the underlying molecular targets that may be injured by exposure to exogenous chemicals during pregnancy.
MATERIALS AND METHODS
Animals and Treatment
All the animal treatments were conducted with the approval of the Institutional Animal Care and Use Committee of the University of Rochester School of Medicine and Dentistry. Animals were housed in microisolator units, had access to food and water ad libitum, and were maintained on a 12L:12D cycle. Wild-type C57BL/6 (Ahrb) mice were either bred in-house or purchased (National Cancer Institute, Frederick, MD). A breeding stock of AHR-deficient mice (B6.129-Ahrtm1G°nz, AhrKO), was obtained from the National Cancer Institute [28]. A breeding stock of Ahrdbd/dbd mice was generously provided by Dr. Christopher Bradfield (University of Wisconsin, Madison, WI). Ahrdbd/dbd mice carry a deletion of the DNA binding domain in the Ahr gene such that they express AHR protein that can bind ligand and migrate to the nucleus, but cannot bind to DNA [29]. Although Ahrdbd/dbd mice have been backcrossed onto a C57BL/6 background, the Ahrdbd/dbd lineage carries the Ahrd allele. This allele encodes a protein with 10-fold lower binding affinity for TCDD; thus, mice on an Ahrd allele background require a 10-fold higher dose of TCDD than the Ahrb allele WT mice in order to elicit similar effects [30]. For some experiments, heterozygous (Ahr+/−) mice were bred to produce age-matched homozygous null (AhrKO) and WT offspring. Colonies of B6.Ahrd, AhrKO, and Ahrdbd/dbd mutant mice are maintained in-house. Female offspring were genotyped at 15 days of age using DNA extracted from an ear punch (QIAamp DNA mini kit; Qiagen, Valencia, CA). PCR was carried out with Gene Amp XL PCR kit (Applied Biosystems, Foster City, CA) using AHR sense and antisense primers according to previous protocol for genotyping AhrKO [31] and Ahrdbd/dbd mice [29].
For timed-pregnant mice, virgin females were placed with males and checked daily for a vaginal plug. The day of the vaginal plug was considered the day of pregnancy 0 (DP 0), and females were then housed individually for the duration of the experiment. In order to analyze the development of mammary glands of age-matched WT, AhrKO, and Ahrdbd/dbd mice, animals were mated between 9 and 11 wk of age and tissue was collected on the day of parturition.
TCDD (≥98% purity; Cambridge Isotopes Laboratory, Andover, MA) was dissolved in 0.1% anisole and diluted in peanut oil. The vehicle control consisted of an equivalent concentration of anisole in peanut oil. Impregnated animals were gavaged with 5 μg/kg or 50 μg/kg body weight of TCDD (Ahrb and Ahrd allele mice, respectively) or peanut oil vehicle control. The half-life of TCDD in C57BL/6 mice is approximately 8–10 days [32]; thus, in order to maintain AHR activation throughout pregnancy, TCDD was administered every 7 days (i.e., on DP 0, DP 7, and DP 14). Animals were killed on DP 6 or on the day of parturition, and mammary glands were removed for analyses. The administered dose of 5 μg/kg of TCDD to mice with the b-allele of the AHR is well below the LD50 of 296 μg/kg determined for C57BL/6 mice [33]. Moreover, this dose range (5–20 μg/kg) is widely used in mouse studies designed to understand the mechanisms by which AHR affects in vivo physiology.
Mammary Gland Transplantation
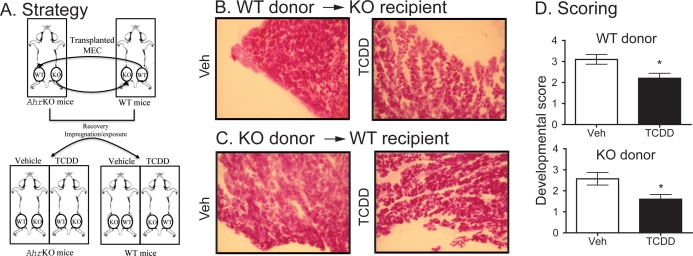
Transplantation of the mammary epithelium was carried out as previously described [34]. Briefly, female AhrKO and WT mice (19–21 days old) were anesthetized with isofluorane. An L-shape small incision was made between the fourth and fifth nipple, and the epithelial tissue from the fourth abdominal right gland from each mouse was identified and removed. The newly cleared fat pad of the AhrKO mouse was then immediately implanted with a small piece (0.5–1 mm diameter) of the WT epithelium, and vice versa. Six to eight wk after surgery (9–11 wk of age), transplant recipients were housed with males for impregnation and exposure to TCDD or vehicle (see Fig. 4A).
FIG. 4.
Impaired development is a consequence of both direct and indirect effects on mammary epithelium. A) A schematic representation of the MEC transplantation strategy is provided, with details in the Materials and Methods section. Animals were mated when they were 9–11 wk of age, and tissue was collected on the day of parturition. B) Representative whole mounts of WT glands transplanted into AhrKO recipients exposed to vehicle (Veh) or TCDD during pregnancy and collected on the day of parturition are shown in the top row. C) Representative whole mounts of AhrKO glands that were transplanted into WT recipients, which were then exposed to Veh or TCDD and impregnated, are shown in the bottom row. Representative images were obtained using a Zeiss dissecting microscope (3.1× magnification) with a Nikon Coolpix E995 camera. All images are at the same magnification. D) The relative development of transplanted glands on the day of parturition was evaluated by at least two scientists and scored on a 1–4 scale. The bar graphs depict the average (±SEM) score of exogenous glands, and the asterisks indicate statistically significant differences between treatment groups of the same genotype (P < 0.05, n = 9). Development of transplanted mammary epithelium from WT and KO mice in vehicle-treated recipient mice did not differ statistically.
Ex Vivo Mammary Gland Development
Female C57BL/6 virgin mice (23–25 days old) were implanted subcutaneously with pellets containing a mixture of estrogen, progesterone, and cholesterol at a ratio of 1:1000:2002 (Innovative Research of America, Sarasota, FL). Fifteen days later, the abdominal and thoracic mammary glands were collected and incubated as previously described [35, 36]. Glands remained floating on the top of a siliconized lens-cleaning tissue (105; Whatman, Maidstone, U.K.) in Waymouth medium containing 100 IU/ml penicillin and 100 μg/ml streptomycin (Gibco Invitrogen Cell Culture, Carlsbad, CA), 20 mM Hepes, and a lactogenic mix (LM) containing the following: bovine insulin (5 μg/ml), aldosterone (100 ng/ml), hydrocortisone (100 ng/ml), mouse epidermal growth factor (60 ng/ml), sheep prolactin (1 μg/ml), and 0.1% bovine serum albumin. All the proteins and hormones used in the LM were obtained from Sigma-Aldrich (St. Louis, MO). In addition to LM, glands were exposed to either 10 nM TCDD diluted in dimethyl sulfoxide (DMSO) or 0.1% DMSO as vehicle control. Right thoracic glands served as controls for preincubation development. Following 6 days in culture, whole mounts were prepared from the left abdominal glands and analyzed for morphological development. The right abdominal glands were stored at −80°C for protein analysis.
Morphological Development Analyses
Mammary gland whole mounts were prepared as described previously [9, 12]. Briefly, evaluation of mammary development was performed without knowledge of treatment by at least two different scientists. Glands were given a developmental score based on a four-point scale (1 = poor development/differentiation to 4 = excellent growth and development). The subjective scoring scales were specific to the stage of development. For glands collected on the day of parturition, development was evaluated according to the quality and quantity of lobuloalveolar units. In addition to a subjective scoring, the assessment of the tissue obtained from ex vivo incubation was performed based on the number and quality of lobuloalveolar and branching morphogenesis. The quantitative morphometric analysis of postincubation tissues was made in two sections that were randomly selected from different regions of photographed mammary gland whole mounts. Digital micrographs were evaluated in a blinded mode. The number of lobuloalveolar units was determined using printed images representing 1.56 mm2 of tissue. The number of branches in a total linear length of about 6 mm of duct was evaluated using a PlanWheel SA2 (Scalex Corporation, Carlsbad, CA). Mean scores for each group were computed and analyzed for differences due to treatment.
Western Blots
Mammary tissue was homogenized in buffer containing 10 mM Hepes, 1 mM ethylenediaminetetraacetic acid (EDTA), 150 mM NaCl, 0.6% NP-40, 0.1 M phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Protein concentration was determined, and 25 μg of protein from each sample was subjected to SDS-PAGE and then transferred to nitrocellulose membranes. Beta-casein protein was visualized by probing with a goat polyclonal anti-mouse β-casein antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) followed by horseradish peroxidase-conjugated donkey anti-goat antibody (Santa Cruz Biotechnology, Inc.). Antibodies to β-actin (Sigma Chemical Co., St. Louis, MO) were used as a control. Antibody complexes were visualized using enhanced chemiluminescent reagents (Amersham Pharmacia, Piscataway, NJ).
Single Cell Suspensions from Mammary Glands
To isolate MECs, we pooled the second and third thoracic and the fourth abdominal glands of three animals per sample; the glands were collected in such a way that lymph nodes were excised prior to preparing single cell suspensions, which was done as previously described [37]. Briefly, dissociation was performed by mincing the tissue in DMEM/F12 media containing 5% fetal bovine serum (FBS) (Gibco Invitrogen), 300 U/ml collagenase, and 100 U/ml hyaluronidase mix (StemCell Technologies, Vancouver, Canada), followed by 2-h incubation on a rocking tray at 37°C. Red blood cells were eliminated using an ammonium chloride lysing solution (0.15 M NH4Cl, 10 mM NaHCO3, 1 mM EDTA). A single cell suspension was then obtained by sequential enzymatic treatments with 0.25% trypsin-EDTA (Gibco Invitrogen) followed by 5 mg/ml dispase II (StemCell Technologies) and 0.1 mg/ml DNase I (StemCell Technologies). All the enzymatic reactions were interrupted by the addition of cold Hanks balanced salt solution modified (HBSS) supplemented with 2% FBS and filtered through a 40-μm cell strainer. After the final centrifugation, cells were resuspended in 1 ml HBSS, and the number of cells was determined using a Coulter Counter (Beckman Coulter, Brea, CA).
Fluorescence-Activated Cell Sorting
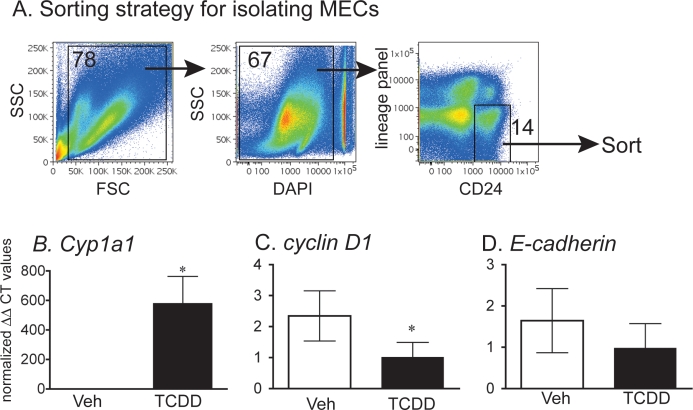
Single cell suspensions were incubated in a solution containing 50 μg/ml rat immunoglobulin G and 5 μg/ml of anti-mouse CD16/32 antibody for 15 min prior to staining with antibodies. The following antibodies were used: anti-mouse CD24 (clone M1/69) conjugated to PerCP-Cy5.5 was used as a marker for epithelial cells and a hematopoietic lineage panel containing biotinylated anti-mouse CD3 (clone 145–2C11), CD45/B220 (clone RA3-6B2), CD11B (clone M1/70), erythroid marker (clone TER-119), LY6G (clone RB6-8c5), and CD31 (PECAM1, clone 390, 13–031) was used to exclude nonepithelial cells. Fluorescein isothiocyanate-conjugated streptavidin was added to label biotinylated antibodies. All the antibodies were purchased from eBioscience (San Diego, CA). Dead cells were identified by the addition of 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA). DAPI−Lin−CD24+ cells were sorted using a FACSAria (BD, Franklin Lanes, NJ) and used for RNA extraction (see Fig. 5).
FIG. 5.
TCDD-induced changes in gene expression in isolated MECs. A) The overall strategy for fluorescence-based sorting (isolation) of MECs is depicted. The number on each dot plot indicates the percentage of cells in the gated region. The preparation of single cell suspensions, antibody binding, and gating are described in detail in Materials and Methods. Bar graphs (B–D) represent average (±SEM) gene expression levels in MECs isolated from mammary glands collected from animals exposed to TCDD or vehicle on DP 0 and killed on DP 6. Each sample is derived from tissues that were pooled from three different animals. Lymph nodes were not included in the tissue preparation. Averages were calculated using the ΔΔCt approach and normalized against GAPDH and vehicle control. Bars with asterisks indicate statistically significant differences (P < 0.05, n = 3–4/group). Original magnification ×3.1 (B, C).
RNA Extraction and Real-Time PCR
RNA was isolated and purified from whole tissue with Ribopure kit (Applied Biosystems/Ambion, Austin, TX) and from isolated MECs with RNAqueous (Applied Biosystems/Ambion) following the instructions provided with the kits. When the abdominal glands were used, lymph nodes were excised and not used in the tissue preparation or extraction of nucleic acids. Total RNA was quantified using a nanodrop (Thermo Scientific, Wilmington, DE), and the reverse transcriptase (RT) reaction was performed with oligo dT, followed by enzymatic reaction with M-MLVRTase (Applied Biosystems/Ambion). Real-time PCR was performed for Cyp1a1 (Forward: TTT GGA GCT GGG TTT GAC AC; Reverse: CTG CCA ATC ACT GTG TCT A) (IDT Technologies, Coralville, IA), insulin-like growth factor 1 (size band: 98, reference position 1322; SABiosciences, Frederick, MD), E-cadherin (size band: 186, reference position: 3783; SABiosciences), cyclin D1 (size band: 172 reference position: 3249; SABiosciences), and SYBR green supermix (Biorad, Hercules, CA). Amplification was performed on an iCycler MyiQ2 (Biorad) using triplicate reactions. GAPDH and L13 were used as internal control reference genes, and differences in gene expression were calculated using the ΔΔCt method [38].
Statistical Analyses
Data were analyzed using StatView software (SAS Software, Cary, NC). Using a two-way ANOVA followed by posthoc tests (Bonferroni/Dunn test), differences between independent variables were compared over time and between treatment groups. Differences between two groups at a single point in time were evaluated using a Student t-test. Differences were considered significant when P values were <0.05.
RESULTS
TCDD Alters Mammary Gland Development Through Activation of the AHR and Its DNA-Binding Domain
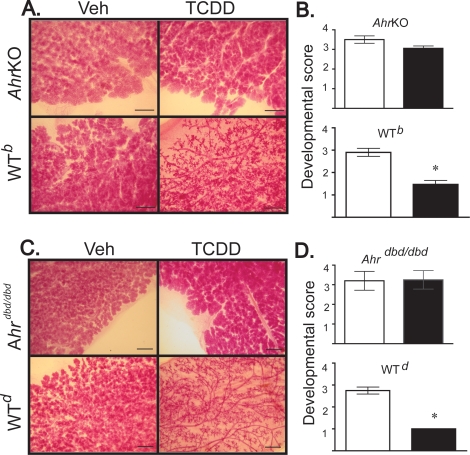
The present data demonstrate that both AHR protein and its DNA-binding domain are required for the deleterious effects on pregnancy-induced mammary gland development caused by exposure to TCDD. However, neither is required for the normal development of the mammary gland. As shown in Figure 1, pregnant WT animals exposed to TCDD demonstrated a profound defect in pregnancy-associated mammary development. When compared to the vehicle treatment group, mammary glands collected from TCDD-treated WT animals had stunted growth; alveolar structures appeared poorly formed and empty. Scoring of glandular development revealed scores that were at least 50% lower than the vehicle-treated group (Fig. 1, A–C). These changes correlated with a substantially reduced expression of β-casein (data not shown). In contrast, when TCDD was administered to AhrKO or Ahrdbd/dbd mice (Fig. 1, A and B, respectively), none of the above defects were noted. In addition, compared to mammary glands collected from vehicle-treated WT animals, mammary glands from AhrKO and Ahrdbd/dbd mice appeared to have normal development during pregnancy, that is, on the day of parturition, mammary glands from AhrKO and Ahrdbd/dbd mice had alveolar structures that were completely formed, filled with milk, and fully populated the fat pad. Moreover, these mice were able to nutritionally support their offspring. Thus, AHR does not appear to be required for the normal development of mammary glands during pregnancy, but the deleterious effects of TCDD on tissue growth and differentiation are AHR-mediated and require AHR binding to DNA.
FIG. 1.
The effects of TCDD on mammary development are mediated through the AHR and require its DNA-binding domain. Nulliparous wild-type (WT), homozygous Ahr−/− (AhrKO), or AhrKOdbd/dbd mice (age 9–11 wk) were impregnated and dosed with TCDD as described in Materials and Methods. All the animals were killed on the day of parturition, tissue was collected, and whole mounts were prepared for the evaluation of development. Representative whole mounts of mammary glands from (A) vehicle and TCDD-treated AhrKO and WTb (b-allele of Ahr), and (C) AhrKOdbd/dbd and WTd (d-allele) show pregnancy-induced differentiation. Representative images were obtained using a Zeiss dissecting microscope (3.1× magnification) with a Nikon Coolpix E995 camera. B, D) Average developmental scores were determined. Error bars indicate the SEM, and asterisks indicate statistically significant differences between vehicle- and TCDD-treated mice of the same genotype (P < 0.05, n = 2–12). Data are representative of two separate experiments.
TCDD Exposure Alters Expression of Genes on Mammary Tissue
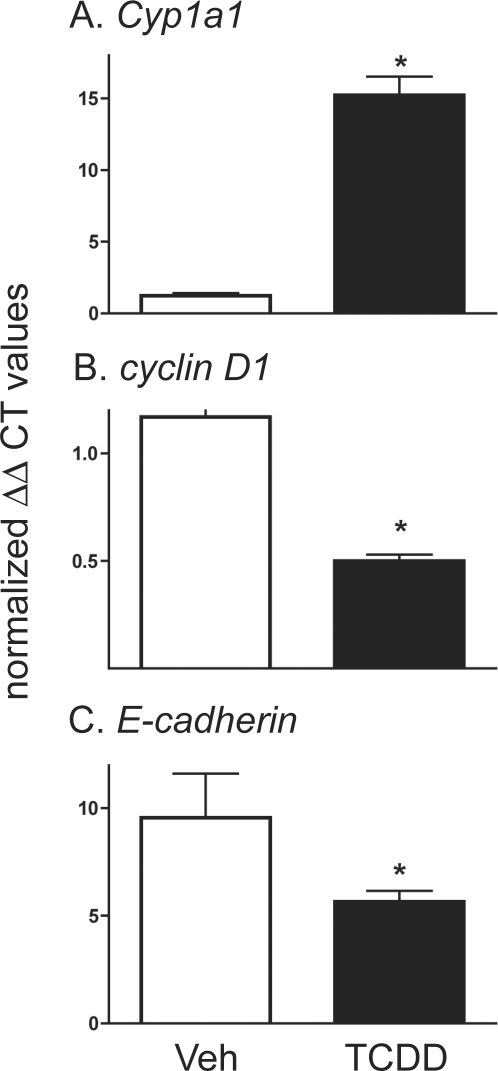
Given that AHR is a transcription factor, that mammary tissue expresses AHR [39–41], and that we have previously reported that TCDD treatment reduces MEC proliferation and differentiation [9], we examined whether AHR activation alters the expression of genes related to cell cycle progression and differentiation in early pregnancy (DP 6). While the expression of many genes was unaffected by TCDD treatment, when compared to tissue collected from animals treated with the peanut oil vehicle, AHR activation by TCDD exposure induced a 12-fold increase in the expression of the well-characterized AHR target gene Cyp1a1 (Fig. 2A). In contrast, cyclin-D1 and E-cadherin expression levels were reduced 2- to 3-fold in glands from TCDD-exposed animals (Fig. 2, B and C, respectively). These alterations in gene expression suggest that AHR activation by TCDD impairs lactogenic differentiation, at least in part, by altering the production of molecules involved in tissue growth. However, molecules produced within the mammary gland and signals derived from other organs control expression of these factors; thus, these changes could be the result of primary AHR targets in the endocrine system, mammary fat pad, or in the mammary epithelium.
FIG. 2.
AHR activation during pregnancy alters gene expression in mammary tissue. Bar graphs represent average (±SEM) level of gene expression in mammary glands collected from WT animals exposed to 5 μg/kg TCDD or vehicle (Veh) on DP 0 and killed on DP 6. Average levels of gene expression were calculated using the ΔΔCt method and normalized against L13 and vehicle control group. The graphs represent the relative expression of the following genes: (A) Cyp1a1, (B) cyclin D1, and (C) E-cadherin. Asterisks indicate statistically significant differences (P < 0.05, n = 6).
TCDD Impairs Mammary Tissue Development by Direct Effects
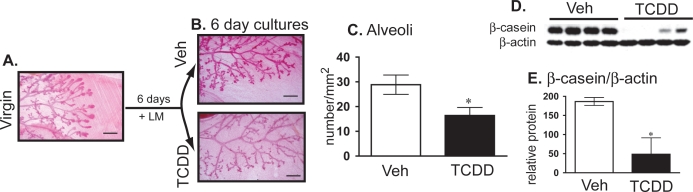
To determine whether exposure to TCDD affects pregnancy-associated mammary development by acting directly on the mammary gland, we isolated glands from virgin mice and induced differentiation of the whole organs in culture [35]. When mammary tissue was incubated with lactogenic stimuli, the glands developed clearly noticeable secondary and tertiary branching, the majority of the stromal tissue was covered by lobule-alveoli structures (Fig. 3, A and B), and β-casein expression was induced (Fig. 3E, left side). However, when TCDD was added to the cultures, glandular development was notably stunted (Fig. 3B). Secondary and tertiary branches could be observed, but there were on average about 40% fewer lobule-alveoli structures than in the control group (Fig. 3C). In addition, the levels of β-casein were significantly decreased in glands treated with TCDD (Fig. 3, D and E). These results indicate that TCDD impairs mammary gland development through direct action on mammary tissue. However, this does not rule out the possibility of additional contributions on pregnancy-associated glandular differentiation due to AHR-mediated actions on other organs and tissues.
FIG. 3.
Ex vivo TCDD exposure impairs mammary development by direct effects on the mammary tissue. Representative images of mammary tissue (3.2× magnification) from glands removed from the following groups: (A) virgin (25–28 days old) animals before culture and (B) after culture for 6 days with LM containing 0.1% DMSO (Veh) or TCDD (10 nM). C) Bars represent the average number of alveolar structures on mammary glands exposed in culture to 0.1% DMSO (Veh) or TCDD (10 nM). D) Immunoblots show levels of β-casein, a marker of mammary gland differentiation, in tissue homogenates from WT animals. Immunoblots were also probed with an antibody to β-actin, which served as a loading control. Each lane represents tissue from a different animal. E) The bar graphs depict results of densitometric analysis of the average β-casein levels. Tissue collected from nonprimed animals served as control for ex vivo differentiation, and they did not develop under any condition (data not shown). In addition, we cultured mammary glands collected from AhrKO mice. Lack of AHR did not alter tissue development in this system, and ex vivo cultured AhrKO glands were not affected by TCDD treatment (data not shown). Data represent mean ± SEM (n = 9; P < 0.05 compared to vehicle control). Results are representative of at least two independent experiments.
Impaired Development Is a Consequence of Direct and Indirect Effects on the Mammary Epithelium
We next sought to determine whether activation of the AHR causes these changes through a direct or indirect impact on the mammary epithelium. To accomplish this, we reciprocally transplanted AhrKO epithelium into cleared fat pads of WT animals, and vice versa, to create WT mice with AhrKO epithelium and AhrKO animals with WT epithelium. Therefore, in the WT recipient animals, all the organs and the mammary stroma are responsive to TCDD, but the transplanted epithelium is nonresponsive (AhrKO, Fig. 4C). On the other hand, in AhrKO recipients all the organs and glands, including the mammary fat pad tissue, are nonresponsive; however, the transplanted mammary epithelium is responsive to TCDD (WT, Fig. 4B). After recovery from surgery, transplant recipients were impregnated and exposed to TCDD or vehicle control, and mammary glands were collected on the day of parturition (Fig. 4D). Both WT and AhrKO transplanted glands exposed to TCDD during pregnancy had their development impaired to some extent, with the average developmental scores significantly lower than they were for glands collected from animals exposed to vehicle treatment (Fig. 4, B and C). However, compared to the consequences of AHR activation in WT animals, the effects of TCDD on reciprocal transplants appear less pronounced (compare the images in Fig. 1A with Fig. 4, B and C). These observations suggest that TCDD exposure impairs pregnancy-associated mammary development by direct AHR-mediated effects on the epithelium and through AHR-mediated effects in the stromal tissues, and possibly via effects in other organs and glands as well.
Exposure to TCDD Alters the Expression of Genes in Isolated MECs
To examine the correlation between alterations in gene expression observed in whole tissue (Fig. 2) and epithelium, we isolated MECs from animals exposed to TCDD on DP 0 and killed on DP 6 (Fig. 5A). In isolated MECs, there was a 300-fold increase in Cyp1a1 gene expression after exposure to TCDD, consistent with other reports that AHR ligands induce Cyp1a1 expression directly in mammary cells [39, 42, 43]. Interestingly, while expression of cyclin D1 was decreased in MECs isolated from mice exposed to TCDD, the TCDD-induced reductions in E-cadherin expression level was not observed in isolated MECs (Fig. 5B). These results suggest that exposure to TCDD altered the expression of cyclin D1 within MECs, which is of interest as this is an important factor in cell cycle progression. The lack of effect of TCDD on E-cadherin expression levels in isolated MECs suggests that alterations in E-cadherin after AHR activation may be related to AHR-mediated cues from cells of the mammary fat pad or other signals extrinsic to MECs.
DISCUSSION
The present study demonstrates that the deleterious consequences of TCDD on pregnancy-associated mammary development and lactogenesis occur through local effects on the mammary gland and that AHR activation in both the epithelial parenchyma and in the associated stromal tissue are required for this impairment. Understanding how environmental pollutants, such as dioxins, influence pregnancy-associated mammary gland development provides greater insight into two important processes: pregnancy-associated mammary gland differentiation and AHR-regulated development. Synchronized and controlled development of mammary glands during pregnancy is necessary for appropriate milk production. Reasons for lactation insufficiency are not well understood; however, decreased milk production as a consequence of exposure to pollutants has been proposed as a contributing factor leading to difficulties in initiating or maintaining breastfeeding [8, 10]. Yet few studies have been undertaken to determine precisely how environmental toxicants affect mammary gland differentiation during pregnancy. In addition to helping understand how chemicals from our environment disrupt this process, studies of mammary gland development during pregnancy provide insight into how AHR regulates complex developmental processes. Mammary gland development during pregnancy follows a pattern of proliferation and differentiation that is quite similar to events that occur in other organs during prenatal development [4, 27]. Thus, processes that occur in other organs during fetal development can be studied in an adult animal simply by impregnating the female. Herein we used several different experimental models to better understand how AHR activation during pregnancy deregulates mammary development and impairs lactogenesis.
In addition to responding to xenobiotics, the AHR has been implicated as an important regulator of growth and development; however, the specific nature of AHR's physiological functions remains enigmatic [13, 17]. AHR activation by exogenous ligands disrupts proliferation and tissue differentiation in many experimental systems, and AHR-null mice have defects in hepatic growth, decreased fertility, ovarian changes, and abnormalities with aging [18–21]. However, the role of AHR in normal mammary gland development remains controversial. We show here that lack of AHR did not alter normal mammary gland development, and homozygous AHR-deficient dams were able to nutritionally support their offspring. This is consistent with another report wherein AHR-null and ARNT-null mice were able to nurse their litters normally [24]. Collectively, these data suggest AHR and its dimerization partner are not required for mammary gland development and lactation. However, others have reported that mammary glands from AHR-null mice showed impairment in branching and morphogenesis in an ex vivo culture system [23]. These differences may arise because of distinctions in experimental procedures, physiological setting (in vivo vs. in vitro), endpoints measured, or mouse strain. For example, the report by Hushka et al. [23] used AHR-null mice that were created by one group, while in our experiments we used AhrKO mice created in a different manner. Also, we examined this process in vivo, whereas they used an ex vivo system for differentiating the tissue. Despite such differences in studies with AhrKO mice, it remains clear that AHR activation by exogenous ligands has a profound impact on mammary gland differentiation.
Mammary gland development during pregnancy is controlled by many factors that are produced systemically and locally [4–6]. Using several experimental approaches, we endeavored to tease apart which tissues and cell types are directly affected by TCDD. Ex vivo culture of whole organs revealed that TCDD acts directly on mammary glands, which is consistent with a prior report that mammary explants treated with 2,3,7,8-tetrachlorodibenzofuran (another potent AHR ligand) displayed decreased lobule development [23]. Likewise, direct treatment of cultured mammary epithelial cells with TCDD impairs differentiation and β-casein induction [39]. Collectively, these findings in mammary tissues and cells are consistent with reports showing that AHR ligands directly influence the differentiation of other organs [29, 44–48]. Yet, organs are made up of different cell types, making it somewhat difficult in many systems to delineate the specific subsets of cell types within an organ that are responsible for AHR-mediated changes in differentiation. It is possible to distinguish AHR-mediated events in mammary epithelium and stromal tissues by reciprocal transplantation, which is an approach that has been used to delineate the contribution of receptors and signaling molecules in other studies [49–51]. Reciprocal transplantation of AHR+/+ and AHR−/− mammary epithelium revealed that local factors produced by the mammary epithelium and stromal tissues (fat pad) both contain direct AHR targets, which are involved in disrupting pregnancy-associated differentiation. Future experiments using transgenic systems to conditionally eliminate AHR in specific mammary cell types would ultimately clarify whether epithelial or stromal AHR in the mammary gland explains the observed changes in pregnancy-associated differentiation.
In addition to demonstrating that AHR has targets within and extrinsic to the mammary epithelium, we present here the novel finding that whatever signaling events are triggered by TCDD exposure requires the AHR's DNA-binding domain. This further supports the idea that AHR regulates mammary gland differentiation at the level of transcriptional control. One potential target gene is cyclin D1, which showed reduced expression in whole mammary glands and sorted MECs from TCDD-treated mice. Given that AHR activation decreases MEC proliferation in early pregnancy, decreased cyclin D1 is consistent with a defect in proliferation [9]. Of course, one possible explanation for reduced cyclin D1 is that that there are simply fewer MECs. However, many genes were not changed by TCDD treatment (data not shown), and other genes, such as Cyp1a1, were markedly elevated by TCDD exposure. There are two additional pieces of evidence to suggest a relationship between AHR signaling and cyclin D1. First, the work of others has shown that activated AHR inhibits proliferation and induces cell cycle arrest in other types of cells and tissues; thus, observing this in mammary glands is consistent with these studies (reviewed in [17, 52]). In particular, AHR activation by TCDD decreased cyclin D1 in prostate cancer cells and breast cancer cell lines [53, 54] as well as in mouse liver [45] and zebrafish caudal fin regeneration models [55]. The second piece of evidence stems from reports that cyclin D1 is essential for mammary gland development during pregnancy [56, 57]. Loss of cyclin D1 leads to a paucity of alveolar cells, which fail to functionally differentiate. In fact, mammary gland development during pregnancy is stunted in cyclin D1-deficient mice with a phenotype that looks strikingly similar to TCDD-treated mice. Thus, an AHR-mediated diminution in cyclin D1 provides a possible mechanism for reduced pregnancy-associated MEC proliferation and may explain in part the decreased number of proliferating mammary epithelial cells in TCDD-treated pregnant mice. However, an impact on cyclin D1 does not rule out additional affects of TCDD on growth factors or other regulatory molecules that are locally produced in the mammary gland.
Indeed, we also noted that mammary glands from TCDD-treated mice had reduced expression of E-cadherin, which is a cell-adhesion molecule directly involved in and crucial for the development and differentiation of epithelial cells in various tissues, including the mammary gland [58]. Other reports have shown that AHR activation reduces E-cadherin protein levels in whole mammary glands, MCF-7 cells, and SCp2 cells, suggesting a direct effect of TCDD on MEC and on this gene in particular [40]. However, when we isolated MECs from TCDD-treated pregnant mice, we did not observe decreased E-cadherin expression levels. These contradictory results may be due to the fact that the cell lines were cultured with other factors and/or on Matrigel, which provides an exogenous extracellular matrix. Timing may also be a factor in sensitivity of E-cadherin to perturbation by AHR activation. In our prior work, we examined E-cadherin in late pregnancy (DP 17) and in SCp2 mammary epithelial cells, which were clonally derived from mice at midpregnancy [59]. In contrast, in our present study, MECs were isolated from animals exposed to TCDD in vivo during early pregnancy (DP 0–6). Thus, E-cadherin expressed by MECs during early pregnancy may not be altered by TCDD exposure, whereas AHR may alter E-cadherin expression in later stages of pregnancy. Alternatively, AHR-mediated changes in E-cadherin levels may be downstream of a direct impact of AHR on other gene targets and signaling pathways, including gene targets that are within mammary tissue but not that are extrinsic to the epithelial compartment.
The new findings presented here suggest that impairment of pregnancy-associated mammary gland development after exposure to TCDD occurs due to AHR-mediated alterations in the normal function of the mammary epithelium and mammary fat pad (stroma). In addition to providing new information regarding how AHR ligands alter this orchestrated developmental process, these findings demonstrate that mammary tissues are directly targeted by a common environmental toxicant in a manner that impedes lactogenesis. When considering public health, the implications of this are profound. Decreased milk production as a consequence of exposure to environmental pollutants may contribute to poor nutrition, especially in places where breast milk is the only food available for the neonate and where exposures to pollution remain poorly controlled. In addition to human health, wild animal populations are exposed to dioxins and related chemicals, which may result in decreased milk production in these species. Another possible implication of this work is a potential connection to breast cancer. Although not directly examined in the work described herein, several molecules involved in normal mammary gland development and differentiation have been implicated in the formation of mammary tumors [60]. This possible relationship is further suggested by the fact that TCDD is a known human carcinogen, and a relationship between exposure to TCDD and related AHR-binding pollutants and breast cancer has been reported [61–63]. Thus, in addition to improving our understanding of how AHR signaling and exogenous AHR-binding pollutants influence lactogenesis, the results from the present study suggest possible involvement of AHR ligands in the development of breast cancer.
ACKNOWLEDGMENTS
We thank Dr. Suzanne E. Fenton (NIEHS) for generously sharing her expertise in mammary gland physiology and development, advice on mammary transplant surgeries, and for thoughtful discussion of our findings. We also thank Jill Gresens for excellent technical assistance with the real time RT-PCR and the preparation of mammary images for this manuscript.
Footnotes
Supported by research and training grants from the National Institutes of Health (K02-ES012409, R21-ES013863, R01-ES013958 to B.P.L.), T32-ES07026, and Environmental Health Sciences Center (P30-ES01247) Pilot Project Award (to B.J.L. and B.P.L.). B.J.L. is the recipient of a Seed the Scientist Award from the Art BeCAUSE Foundation.
REFERENCES
- Robinson G, McKnight R, Smith G, Henninghausen L. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development 1995; 121: 2079 2090 [DOI] [PubMed] [Google Scholar]
- Hovey R, Trott J, Vonderhaar B. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mam Gland Biol Neoplasia 2002; 7: 17 38 [DOI] [PubMed] [Google Scholar]
- Neville M, McFadden T, Forsyth I. Hormonal regulation of mammary gland differentiation and milk secretion. J Mam Gland Biol Neoplasia 2002; 7: 49 66 [DOI] [PubMed] [Google Scholar]
- Henninghausen L, Robinson G. Information networks in the mammary gland. Nat Rev Mol Cell Biol 2005; 6: 715 725 [DOI] [PubMed] [Google Scholar]
- Hovey R, McFadden T, Akers R. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mam Gland Biol Neoplasia 1999; 4: 53 68 [DOI] [PubMed] [Google Scholar]
- Neville M, Medina D, Monks J, Hovey R. The mammary fat pad. J Mam Gland Biol Neoplasia 1998; 3: 109 116 [DOI] [PubMed] [Google Scholar]
- Enoch R, Stanko J, Greiner S, Youngblood G, Rayner J, Fenton SE. Mammary gland development as a sensitive end point after acute prenatal exposure to an atrazine metabolite mixture in female Long-Evans rats. Environ Health Persp 2007; 115: 541 547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowsi W, Perara F, Mroz E, Edwards S, Flak E, Rauh V, Pac A, Budzyn-Mrozek D, Musial A. Prenatal exposure to passive smoking and duration of breastfeeding in nonsmoking women: Krakow inner city prospective cohort study. Arch Gynecol Obstet 2008; 278: 411 417 [DOI] [PubMed] [Google Scholar]
- Lew B, Collins LL, O'Reilly MA, BP Lawrence. Activation of the aryl hydrocarbon receptor during different critical windows in pregnancy alters mammary epithelial cell proliferation and differentiation. Toxicol Sci 2009; 111: 151 162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M, Walsh C. Effects of xenobiotics on milk secretion and composition. Amer J Clin Nutr 1995; 61: 687S 694S [DOI] [PubMed] [Google Scholar]
- Soto AM, Vandenberg LN, Maffini M, Sonnenschein C. Does breast cancer start in the womb? Basic Clin Pharmacol Toxicol 2008; 102: 125 133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderstrasse BA, Fenton SE, Bohn A, Cundiff J, BP Lawrence. A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci 2004; 78: 248 257 [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 2008; 21: 102 116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M, Nagy S. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Phramacol Toxicol 2003; 43: 309 334 [DOI] [PubMed] [Google Scholar]
- Gasiewicz TA, Henry EC, Collins LL. Expression and activity of aryl hydrocarbon receptors in development and cancer. Crit Rev Eukaryot Gene Expr 2008; 18: 279 321 [DOI] [PubMed] [Google Scholar]
- Gu Y-Z, Hogenesch J, Bradfield C. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Phramacol Toxicol 2000; 40: 519 561 [DOI] [PubMed] [Google Scholar]
- Puga A, Ma C, JL Marlowe. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol 2009; 77: 713 722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis G, Bradfield CA. AHR null alleles: distinctive or different? Biochem Pharmacol 1998; 56: 781 787 [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Fernandez-Salguero PM. The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metab Dispos 1998; 26: 1194 1198 [PubMed] [Google Scholar]
- Benedict J, T-M Lin, Loeffler I, Peterson R, Flaws J. Physiological role for the aryl hydrocarbon receptor in mouse ovary biology. Toxicol Sci 2000; 56: 382 388 [DOI] [PubMed] [Google Scholar]
- Lund AK, Peterson SL, Timmins GS, MK Walker. Endothelin-1-mediated increase in reactive oxygen species and NADPH oxidase activity in hearts of aryl hydrocarbon receptor (AHR) null mice. Toxicol Sci 2005; 88: 265 273 [DOI] [PubMed] [Google Scholar]
- Cunha G, Cook P, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 2004; 67: 417 434 [DOI] [PubMed] [Google Scholar]
- Hushka L, Williams J, Greenlee W. Characterization of 2,3,7,8-tetrachlorodibenzofuran-dependent suppression and Ah receptor pathway gene expression in the developing mouse mammary gland. Toxicol Appl Pharmacol 1998; 152: 200 210 [DOI] [PubMed] [Google Scholar]
- Le Provost F, Riedlinger G, Yim S, Benedict J, Gonzalez F, Flaws J, Henninghausen L. The aryl hydrocarbon receptor (AHR) and its nuclear translocator (ARNT) are dispensible for normal mammary gland development but are required for fertility. Genesis 2002; 32: 231 239 [DOI] [PubMed] [Google Scholar]
- Henninghausen L, Robinson G. Think globally, act locally: the making of a mouse mammary gland. Genes Dev 1998; 12: 449 455 [DOI] [PubMed] [Google Scholar]
- Henninghausen L, Robinson G. Signaling pathways in mammary gland development. Develop Cell 2001; 1: 467 475 [DOI] [PubMed] [Google Scholar]
- Hovey R, Trott J. Morphogenesis of mammary gland development. Adv Exp Med Biol 2005; 554: 219 228 [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert D, McPhail T, Lee S, Kimura S, Nebert D, Rudikoff S, Ward J, Gonzalez F. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 1995; 268: 722 726 [DOI] [PubMed] [Google Scholar]
- Bunger M, Glover E, Moran SM, Walisser J, Lahvis G, Hsu EL, Bradfield CA. Abnormal liver development and resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in mice carrying a mutation in the DNA binding domain of the aryl hydrocarbon receptor. Toxicol Sci 2008; 106: 83 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol Pharmacol 1994; 46: 915 921 [PubMed] [Google Scholar]
- Takemoto K, Nakajima M, Jujiki Y, Katoh M, Gonzalez F, Yokoi T. Role of the aryl hydrocarbon receptor and CYP1B1 in the antiestrogenic activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Toxicol 2004; 78: 309 315 [DOI] [PubMed] [Google Scholar]
- Gasiewicz T, Geiger L, Rucci G, Distribution Neal R. excretion and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J and B6D2F1 mice. Drug Metab Dispos 1983; 11: 397 403 [PubMed] [Google Scholar]
- Chapman D, Schiller C. Dose-related effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J and DBA/2J mice. Toxicol Appl Pharmacol 1985; 78: 147 157 [DOI] [PubMed] [Google Scholar]
- Young L. The cleared mammary fat pad and the transplantation of mammary gland morpholoical structures and cells. Ip MM, Asch B. (eds.), Methods in Mammary Gland Biology and Breast Cancer Research. New York: Kluwer Academic/Plenum Publishers; 2000: 67 74 [Google Scholar]
- Plaut K, Ikeda M, Vanderhaar B. Role of growth hormone and insulin-like growth factor-1 in mammary development. Endocrinology 1993; 133: 1843 1848 [DOI] [PubMed] [Google Scholar]
- Singh D, DeOme K, Bern H. Strain differences in response of the mouse mammary gland to hormones in vitro. J Natl Cancer Inst 1970; 45: 657 675 [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li H, Eaves C. Purification and unique properties of mammary epithelial stem cells. Nature 2006; 439: 993 997 [DOI] [PubMed] [Google Scholar]
- Schefe J, Lehmann K, Buschmann I, Unger T, Funk-Kaiser H. Quantitative real time RT-PCR data analysis: current concepts and the novel “gene expression's CT difference” formula. J Mol Med 2006; 84: 901 910 [DOI] [PubMed] [Google Scholar]
- Collins LL, Lew BJ, BP Lawrence. TCDD exposure disrupts mammary epithelial cell differentiation and function. Repro Toxicol 2009; 28: 11 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diry M, Tomkiewicz C, Koehle C, Coumoul X, Walter Bock K, Barouki R, Transy C. Activation of the dioxin/aryl hydrocarbon receptor (AHR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene 2006; 25: 5570 5574 [DOI] [PubMed] [Google Scholar]
- Qu X, Metz R, Porter W, Cassone V, Earnest D. Disruption of clock gene expression alters responses of the aryl hydrocarbon receptor signaling pathway in the mouse mammary gland. Mol Pharmacol 2007; 72: 1349 1358 [DOI] [PubMed] [Google Scholar]
- John K, Divi R, Keshava C, Orozco C, Schockley M, Richardson D, Poirier M, Nath J, Weston A. CYP1A1 and CYP1B1 gene expression and DNA adduct formation in normal human mammary epithelial cells exposed to benzo[a]pyrene in the absence or presence of chlorophyllin. Cancer Lett 2010; 292: 254 260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Metz R, Porter W, Neuendorff N, Earnest B, Earnest D. The clock genes period 1 and period 2 mediate diurnal rhythms in dioxin-induced Cyp1A1 expression in the mouse mammary gland and liver. Toxicol Lett 2010; 196: 28 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott B, Held G, Wood C, Buckalew A, Brown J, Schmid J. AHR. ARNT, and CYP1A1 mRNA quantitation in cultured human embryonic palates exposed to TCDD and comparison with mouse palate in vivo and in culture. Toxicol Sci 1999; 47: 62 75 [DOI] [PubMed] [Google Scholar]
- Mitchell K, Lockhart C, Huang G, Elferink C. Sustained aryl hydrocarbon receptor activity attenuates liver regeneration. Mol Pharmacol 2006; 70: 163 170 [DOI] [PubMed] [Google Scholar]
- Latchney S, Lioy D, Henry EC, Gasiewicz TA, Strathmann F, Mayer-Proschel M, Opanashuk LA. Neural precursor cell proliferation is disrupted through activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Stem Cells Dev 2011; 20: 313 326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Borgeest C, Greenfeld C, Tomic D, Flaws J. In utero effects of chemicals on reproductive tissues in females. Toxicol Appl Pharmacol 2004; 198: 111 131 [DOI] [PubMed] [Google Scholar]
- Vezina C, Lin T, Peterson R. AHR signaling in prostate growth, morphogenesis, and disease. Biochem Pharmacol 2009; 77: 566 576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Seagroves T, Kabotyanski E, Hovey R, Vonderhaar B, Lydon J, Miyoshi K, Hennighausen L, Ormandy C, Lee A, Stull M, Wood T, et al. Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloalveolar development. Mol Endocrinol 2002; 16: 2675 2691 [DOI] [PubMed] [Google Scholar]
- Luetteke N, Qiu T, Fenton S, Troyer K, Riedel R, Chang A, Lee D. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999; 126: 2739 2750 [DOI] [PubMed] [Google Scholar]
- Moraes R, Change H, Harrington N, Landua J, Prigge J, Lane T, Wainwright B, Hamel P, Lewis M. Ptch1 is required locally for mammary gland morphogenesis and systemically for ductal elongation. Development 2009; 136: 1423 1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A, Xia Y, Elferink C. Role of the aryl hydrocarbon receptor in cell cycle regulation. Chem Biol Interact 2002; 141: 117 130 [DOI] [PubMed] [Google Scholar]
- Barnes-Ellerbe S, Knudsen K, Puga A. 2,3,7,8-Tetrachlorodibenzo-p-dioixin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol Pharmacol 2004; 66: 502 511 [DOI] [PubMed] [Google Scholar]
- Barhoover M, Hall J, Greenlee W, Thomas R. Aryl hydrocarbon receptor regulates cell cycle progression in human breast cancer cells via a functional interaction with cyclin-dependent kinase 4. Mol Pharmacol 2009; 77: 195 201 [DOI] [PubMed] [Google Scholar]
- Mathew LK, Simonich MT, Tanguay RL. AHR-dependent misregulation of Wnt signaling disrupts tissue regeneration. Biochem Pharmacol 2009; 77: 498 507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 1995; 9: 2364 2372 [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher J, Parker S, Li T, Fazeli A, Gardner H, Haslam S, Bronson R, Elledge S, Weinberg R. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 1995; 82: 621 630 [DOI] [PubMed] [Google Scholar]
- Knudsen K, Wheelock M. Cadherins and mammary gland. J Cell Biochem 2005; 95: 488 496 [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff M, Aggeleer J, Ram T, Bissell M. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membranes. Development 1989; 105: 223 235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanigan F, O'Connor D, Martin FM, Gallagher W. Molecular links bewtween mammary gland development and breast cancer. Cell Mol Life Sci 2007; 64: 3159 3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S, Rowell C, Wang J, Lamartiniere C. Prenatal TCDD exposure predisposes for mammary cancer in rats. Repro Toxicol 2007; 23: 391 396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlezinger J, Liu D, Farago M, Seldin D, Belguise K, Sonenshein G, Sherr D. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem 2006; 387: 1175 1187 [DOI] [PubMed] [Google Scholar]
- Warner M, Eskenazi B, Mocarelli P, Gerthoux P, Samuels S, Needham L, Patterson D, Brambilla P. Serum dioxin concentrations and breast cancer risk in the Seveso Women's Health Study. Environ Health Perspect 2002; 110: 625 628 [DOI] [PMC free article] [PubMed] [Google Scholar]