FIGURE 2.
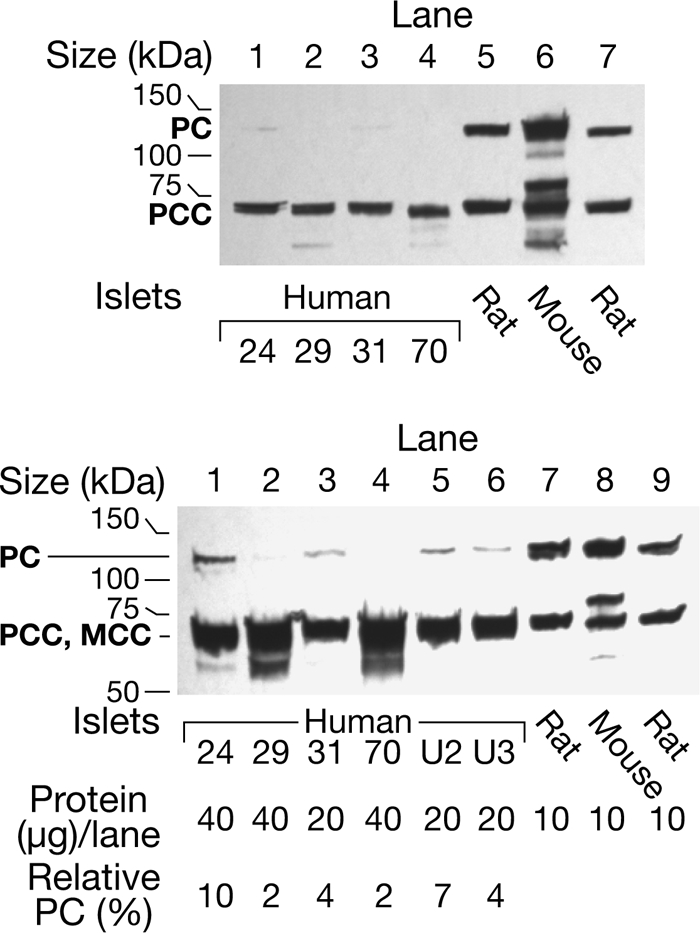
Level of pyruvate carboxylase protein is much lower in human pancreatic islets than in mouse and rat islets. The upper panel shows a streptavidin-probed blot of islets from four human individuals identified by numbers and islets from the rat and the mouse. Each preparation was snap-frozen immediately after isolation. All of these samples happen to have been stored frozen as islet pellets for 8–10 years before they were homogenized in a solution of KMSH containing 1 mm dithiothreitol and analyzed. There was 15-μg whole-cell proteins per lane. The band that migrates at ∼130 kDa is PC and is not visible (lanes 2 and 4) or barely visible (lanes 1 and 3) in the islets from the humans. The band at ∼72 kDa is the α-chain of propionyl-CoA carboxylase (PCC) (with or without methylcrotonyl-CoA carboxylase (MCC) that migrates close to the α-chain of PCC). The density of this band is relatively the same in lanes of the human and rodent islets. The lower panel shows the semiquantification of PC protein and is a streptavidin-probed blot in which larger amounts (20 or 40 μg) of whole-cell protein were added to the lanes containing human islet samples than were added to the lanes containing rodent islet samples. Lanes 1–4 contained the human islets and lanes 7 and 8 contained the rat and mouse islets shown in the top panel. Lanes 5 and 6 contained human islet samples homogenized and boiled in SDS gel sample buffer on the day of receipt and analyzed 1 month and 1 week later, respectively; and lane 9 contained rat islets stored as a frozen homogenate 3 months before analysis. Densitometric quantification of the PC band indicated that the amount of PC protein/μg islet cell protein was 2–10% of the average of the amounts of PC in the three rodent lanes.
