FIGURE 1.
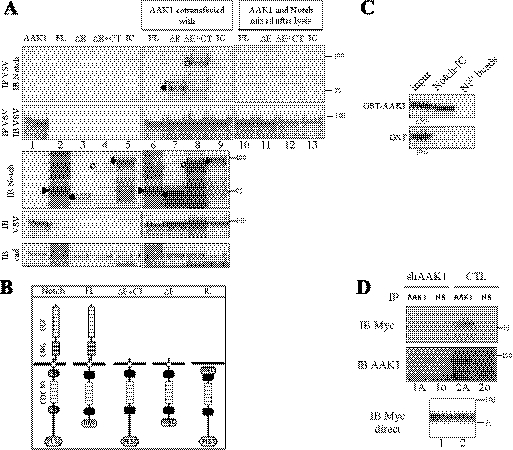
AAK1 directly interacts with the activated form of membrane-anchored Notch. A, AAK1 interacts with Notch in mammalian cells. 293T cells were transiently transfected with plasmids encoding different Notch constructs with or without AAK1 as indicated. Symbols on the left of the lanes indicate the expected Notch forms: Notch FL (furin-cleaved form, ▶), ΔE+CT (○), IC (♦; migrates slower than ΔE+CT because of the 6 N-terminal Myc tags)) or ΔE (●). In lanes 10–13, 500 μg of lysate from AAK1-transfected cells (extract loaded in lane 1) was mixed with 500 μg of lysate from cells transfected with different Notch constructs as indicated (corresponding to extracts loaded in lanes 2–5), and the mixed extracts were immunoprecipitated with anti-VSV. Cell lysates were analyzed by Western blotting for Notch (IB Notch) or AAK1 (IB VSV) following or not immunoprecipitation with anti-VSV (IP VSV). Cadherin was used as a loading control (IB cad); an asterisk indicates a slower migrating form of ΔE and ΔE+CT (lanes 7, 8). B, Notch constructs. Full-length Notch receptor domains are depicted: EGF-, LNG-, ankyrin/CDC10-repeats, NLS, and PEST domains. The ΔE constructs represent extracellular-truncated and constitutively active forms of the receptor, with (ΔE+CT) or without (ΔE) the PEST domain (deleted from amino acids 2186). IC is a construct mimicking the γ-secretase cleavage product of Notch; FL is the inactive full-length receptor, deleted of the C-terminal region including the PEST domain. FL and ΔE constructs are C-terminally truncated; N- or C-terminal 6-Myc tags are indicated. C, AAK1 directly interacts with the Notch intracellular domain. In vitro interaction assay: Purified GST-AAK1 or GST alone were incubated in the presence (Notch-IC) or absence (Ni2+ beads) of immobilized intracellular His-tagged Notch IC. AAK1 binding was detected using an AAK1-specific antiserum (upper panel); GST binding was tested in parallel (lower panel). D, Endogenous AAK1 interacts with Notch ΔE. HeLa cells stably transfected with a vector expressing an shRNA specific for AAK1 (shAAK1) or the empty pSUPER vector (CTL) were transiently transfected with Myc-tagged ΔE. Cell lysates (500 μg) were analyzed after immunoprecipitation with a pre-immune (IP NS, lanes 1o and 2o) or anti-AAK1 serum (IP AAK1, lanes 1A and 2A), followed by immunoblotting for Notch (IB Myc, upper panel) and then reblotted for AAK1 (IB AAK1). A direct Western blot shows the expression of ΔE in 10% of extract (IB Myc direct).