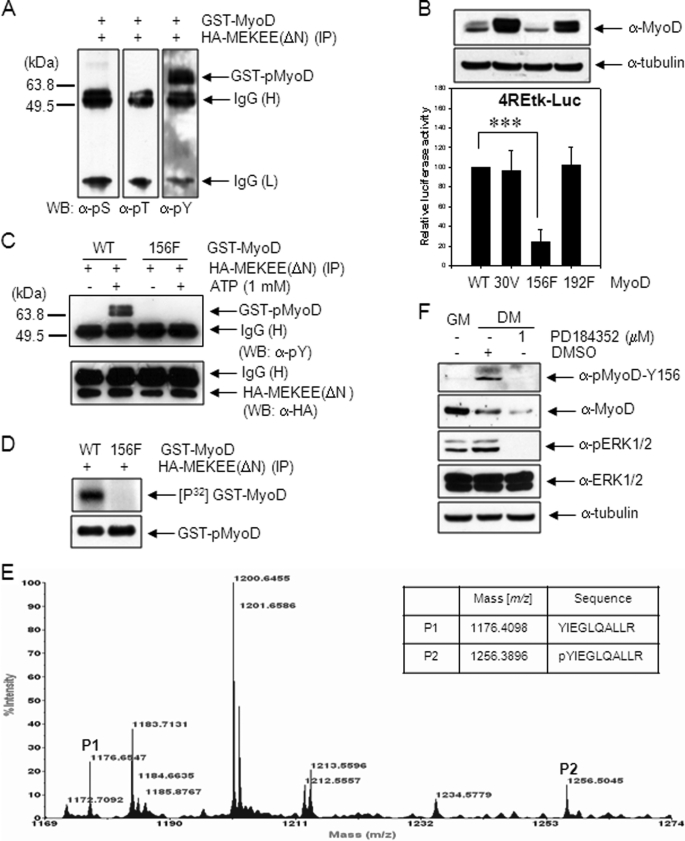
FIGURE 3.
MyoD Tyr-156 is phosphorylated by active MEK1. A, 10T1/2 cells were transfected with HA-MEKEE (ΔN, S218E/S222E). After 36 h, cells were extracted, and the immunoprecipitates prepared with anti-HA antibody were incubated with 2 μg of GST-MyoD protein in a kinase buffer, including 1 mm ATP, at 37 °C for 30 min. The mixtures were immunoblotted using anti-pSer (pS), anti-pThr (pT), or anti-pTyr (pY) antibody. B, 10T1/2 cells were co-transfected with 4REtk-Luc reporter vector and EMSV-MyoD (wild type, Y30V (30V), Y156F (156F), or Y192F (192F)), maintained in DM for 2 days, and assayed for luciferase activity. The expression levels of MyoD proteins were examined by immunoblotting using anti-MyoD (C-20) antibody (upper panel). C, to examine whether recombinant, mutant GST-MyoD-Y156F protein could be phosphorylated by active MEK1, the immune complex prepared in A was incubated with wild type GST-MyoD or mutant GST-MyoD-Y156F protein, and the reaction mixtures were analyzed by immunoblotting using anti-pTyr antibody as described in A. In D, the immune complex was incubated with wild type GST-MyoD or mutant GST-MyoD-Y156F in the presence of 5 μCi of [γ-32P]ATP instead of cold ATP. The reaction mixtures were separated by 10% SDS-PAGE, and the gel was dried and exposed to x-ray film. E, GST-MyoD protein (10 μg) was phosphorylated with immunoprecipitated HA-MEKEE (ΔN) as described previously in A. The tryptic digest of phosphorylated GST-MyoD was analyzed by MALDI-TOF as described under “Experimental Procedures.” The table shows the expected masses of two tryptic peptides, YIEGLQALLR (P1) and pYIEGLQALLR (P2) (ProteinProspector). F, phosphorylation at Tyr-156 of endogenous MyoD in proliferating or differentiating C2C12 cells kept in DM for 3 days was examined by immunoblotting using anti-pMyoD-Y156 antibody. C2C12 cells in lane 3 were treated with PD184352, a MEK inhibitor, for 24 h before cell extraction. The results show the mean ± S.D. of triplicate experiments. ***, p < 0.001. WB, Western blot. H, heavy chain; L, light chain.