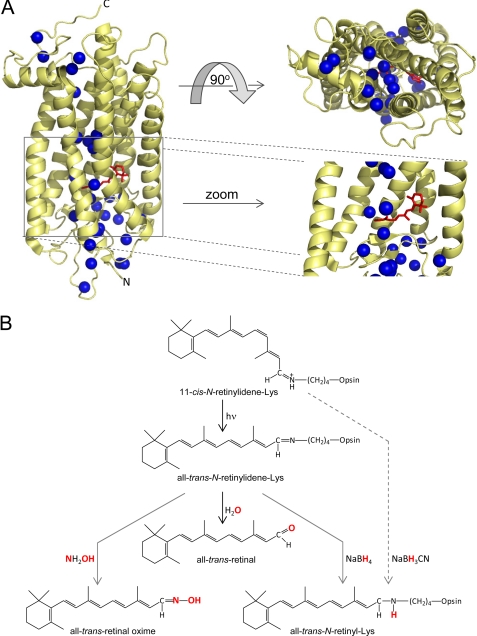
FIGURE 1.
Water molecules present in the crystal structure of bovine Rho and the role of water and small molecules in retinylidene Schiff base cleavage and reduction. A, positions of selected waters (shown as blue spheres) in structures of bovine Rho (Protein Data Bank code 1U19). Several Rho-bound waters are located within the transmembrane helical bundle of the protein. The water network extends from the region of visual chromophore binding (red) to the N and C termini. A magnified view of the chromophore-binding site reveals the presence of oriented water molecules near the retinylidene Schiff base. B, schematic representation of retinylidene Schiff base chemical reactivity in the ground and photoactivated states of Rho. As a consequence of photoreceptor activation by light, the retinylidene-Lys covalent bond is hydrolyzed. In this process, a water molecule donates its oxygen atom to form a new at-RAL molecule. The activated state of Rho allows a small nucleophile (NH2OH) or reducing agents such as NaBH4 to penetrate inside the photoreceptor and chemically modify the Schiff base. Additionally, NaBH3CN was shown to be effective in reducing retinylidene-Lys bound in the ground state of Rho (38).