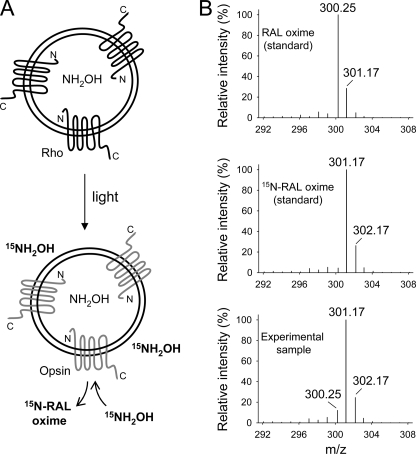
FIGURE 5.
Isotopic composition of at-RAL oximes. Proteoliposomes containing NH2OH were exposed to light in the presence of 15NH2OH added to the surrounding buffer. After extraction, the isotopic composition of at-RAL oxime (syn) liberated from Rho was examined by MS. A, schematic representation of the experimental setup. B, isotope distribution of the at-RAL oxime ([M + H]+) charged state. Upper and middle panels, unlabeled and 15N isotope-containing synthetic standards, respectively; lower panel, isotopic composition of at-RAL oxime (syn) extracted from proteoliposomes exposed to light. Robust incorporation of the 15N isotope into at-RAL oxime released from photoactivated Rho indicates that 15NH2OH derived from the C-terminal side of Rho is solely involved in retinylidene Schiff base cleavage.