Table 1.
Enantioselective, Stereoconvergent Negishi Cross-Couplings of Allylic Chlorides (for the reaction conditions, see Eq 1).
| entry | allylic chloride | R–ZnBr | ee (%) | yield (%) |
|---|---|---|---|---|
| 1 | 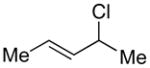 |
 |
90 | 93 |
| 2 |  |
79 | 81 | |
| 3 | 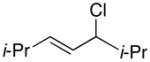 |
69 | 57 | |
| 4 | 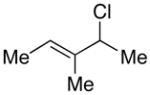 |
 |
98 | 54 |
| 5a |  |
83 | 97 | |
| 6 |  |
 |
84 | 95 |
| 7 |  |
81 | 85 | |
| 8 | 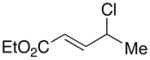 |
 |
96 | 86 |
| 9 | 93 | 91 | ||
| 10 |  |
n-Hex-ZnBr | 90 | 63 |
Regioselectivity: 1.9:1; ee of the minor regioisomer: 88%.
