Table 2.
Using data from placebo groups over 2 years based on 100 subjects in each group to predict the number of expected relapses
| Efficacy | Relapse reduction | 30% | 30% | 50% | 70% |
| Example therapy | IFN-β, GA | terifluonamide | cladribine/fingolimod | natalizumab/mitoxantrone | |
| Side effects (%) | serious | 0 | 0.1 | 0.8/0.8 | 0.1/0.7 |
| ongoing | 80% | minimal | minimal | minimal | |
| Expected relapses with therapy over 2 years | |||||
| Demyelination syndrome subtype (expected relapses in 2 year period) | Rapidly evolving severe (300) | 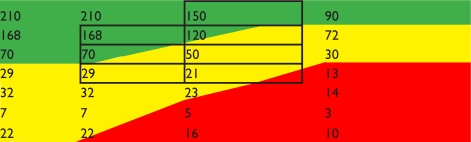 |
|||
| Clinically definite – group 1 (240) | |||||
| Clinically definite – group 2 (100) | |||||
| McDonald (41) | |||||
| CIS ≥ 3 MRI lesions (45) | |||||
| <3 MRI lesions (10) | |||||
| RIS (31) | |||||
Notes: Using trial efficacy data the relapses that will occur as result of therapy can be predicted. Acceptability is highlighted: green, acceptable in most health systems; orange: acceptable in US/parts of Europe; red: not acceptable in health systems due to side effects; grey outline: groups in trials that have been tested.
Abbreviations: CIS, clinically isolated syndrome; RIS, radiologically isolated syndrome; GA, glatiramer acetate.
