Abstract
The food-borne pathogen Listeria monocytogenes proliferates at refrigeration temperatures, rendering refrigeration ineffective in the preservation of Listeria-contaminated foods. The uptake and intracellular accumulation of the potent compatible solutes glycine betaine and carnitine has been shown to be a key mediator of the pathogen's cold-tolerant phenotype. To date, three compatible solute systems are known to operate in L. monocytogenes: glycine betaine porter I (BetL), glycine betaine porter II (Gbu), and the carnitine transporter OpuC. We investigated the specificity of each transporter towards each compatible solute at 4°C by examining mutant derivatives of L. monocytogenes 10403S that possess each of the transporters in isolation. Kinetic and steady-state compatible solute accumulation data together with growth rate experiments demonstrated that under cold stress glycine betaine transport is primarily mediated by Gbu and that Gbu-mediated betaine uptake results in significant growth stimulation of chill-stressed cells. BetL and OpuC can serve as minor porters for the uptake of betaine, and their action is capable of providing a small degree of cryotolerance. Under cold stress, carnitine transport occurs primarily through OpuC and results in a high level of cryoprotection. Weak carnitine transport occurs via Gbu and BetL, conferring correspondingly weak cryoprotection. No other transporter in L. monocytogenes 10403S appears to be involved in transport of either compatible solute at 4°C, since a triple mutant strain yielded neither transport nor accumulation of glycine betaine or carnitine and could not be rescued by either osmolyte when grown at that temperature.
The animal and human pathogen Listeria monocytogenes is the causative agent of listeriosis, a food-borne disease primarily affecting immunocompromised individuals with a 20 to 30% mortality rate (28, 36). L. monocytogenes has long been recognized as an organism capable of proliferating at refrigeration temperatures (46). During the last decade, several aspects of the L. monocytogenes physiology have been identified that are linked to the ability of this otherwise mesophilic pathogen to adapt to environments of low temperature, including the expression of cold shock proteins, the retailoring of the membrane lipid composition, and the accumulation of compatible solutes.
Cold-stress (cold-shock and cold acclimation) proteins whose synthesis is increased after temperature downshifts have been isolated, but for the most part their identities and functions remain uncertain (6, 16, 32, 47). Analogous proteins have been studied for Escherichia coli and Bacillus subtilis and have been postulated to function as RNA chaperons, transcription antiterminators, or transcription activators (9, 13, 14, 19). A recent study of gene expression in response to growth of L. monocytogenes at 10°C showed that the pathogen's acclimation involves amino acid starvation, oxidative stress, aberrant protein synthesis, cell surface remodeling, alterations in degradative metabolism, and induction of global regulatory responses (24). A major and well-characterized aspect of the cryotolerant physiology of Listeria relates to the predominance of low-freezing-point branched-chain fatty acids in the cell membrane, which permits the maintenance of membrane function at low temperatures; the membrane architecture can be further adjusted for growth at low temperatures by the cold-triggered decrease in the fatty-acid chain length and alteration of the fatty-acid branching pattern from iso to anteiso (4, 20, 21, 25-27, 34, 35). The third major element of cryotolerance is the ability of L. monocytogenes to use compatible solutes (osmolytes) as cryoprotectants (23), a property first described for L. monocytogenes and which is now known to be shared by other organisms (8, 15, 17).
Compatible solutes are usually low-molecular-weight and highly soluble compounds that bear no net charge at physiological pH and have been known to function as osmoprotectants for a variety of eukaryotic and prokaryotic organisms (48). The molecular basis of their action as osmoprotectants arises from the fact that they can be accumulated at high levels inside the cell in response to increased external osmolarity, thus restoring turgor, without affecting cytoplasmic functions (48). Glycine betaine, carnitine, acetylcarnitine, γ-butyrobetaine, proline betaine, and 3-dimethylsulphoniopropionate have been shown to confer osmoprotection for L. monocytogenes (7). Glycine betaine cannot be synthesized by Listeria, and its accumulation is mediated by active transport from the environment. Carnitine is likewise accumulated via transport. Two transport systems have been biochemically and genetically identified as glycine betaine transporters (11, 12, 22, 37), and one has been identified as a carnitine transporter (2, 10, 39, 45).
Glycine betaine porter II (Gbu) is an ATP binding cassette (ABC) transporter that is encoded by the gbu operon (22). Transport via Gbu can be activated by increased osmotic pressure or decreased temperature (11, 22). Glycine betaine porter I (BetL) is a secondary transporter that is activated by hyperosmotic gradients and mediates the cotransport of glycine betaine with a sodium ion (i.e., a glycine betaine-Na+ symporter) (12, 37). OpuC is an ABC transporter, the product of the opuC operon that has been shown to transport carnitine in response to osmotic and cold stress (2, 10, 39).
Considerable research has accumulated on the transport of glycine betaine and carnitine by L. monocytogenes in response to hyperosmotic stress (2, 7, 23, 29, 30, 44). The role of the transporters Gbu, BetL, and OpuC has been studied both in single-mutant strains (lacking one transporter) and recently in double- and triple-mutant strains, possessing each transporter in isolation (1), and the ability to transport glycine betaine or carnitine has been compared to that of their respective parent strains. In contrast, very little is known about the role of these transporters under conditions of low-temperature stress. A recent study has demonstrated that an L. monocytogenes mutant lacking the glycine betaine porter Gbu is impaired in the accumulation of betaine but exhibits increased carnitine accumulation, which partially restores cryotolerance in refrigerated milk whey (3). Deletion of the carnitine transporter OpuC also resulted in reduced growth in defined medium (2).
The contribution of each of the porters to solute accumulation and to tolerance to chill stress remains uncertain. Furthermore, whether additional glycine betaine and carnitine transporters operate in L. monocytogenes under chill stress remains unknown. Therefore, the objectives of this work were to determine the role of the L. monocytogenes glycine betaine and carnitine transporters under low-temperature stress; to achieve this goal, we characterized double and triple transporter mutant strains so that the action of Gbu, BetL, and OpuC could be measured without mutual interference. We determined the transporters' specificities for glycine betaine and carnitine, compared the cryoprotective potentials of these transporters in environments where glycine betaine, carnitine, or both solutes are present, and investigated whether additional transporters for either solute operate in L. monocytogenes under cold stress.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
L. monocytogenes 10403S (43) was the parent strain used in this work. It is a common laboratory strain and is hereafter referred to as the wild type. Its mutant derivative strains, ASA4, ASA5, ASA6, and ASA7 (1), were created using the splicing by overlap extension technique (SOE) (18) followed by two-step allele replacement mutagenesis using the L. monocytogenes-E. coli shuttle vector pKSV7 (40). The resulting mutants carry combinations of the following: an internal 681-bp deletion in the betL gene encoding the glycine betaine porter I (SOEbetL25), an internal 1,836-bp deletion in the gbu operon encoding the glycine betaine porter II (SOEgbu10), and an internal 2,517-bp deletion in the opuC operon (SOEopuC15) encoding the carnitine transporter OpuC. ASA4 is a double-mutant strain (SOEbetL25gbu10) possessing the carnitine transporter OpuC but lacking the betaine porters BetL and Gbu; ASA5 is a double-mutant strain (SOEbetL25opuC15) possessing the glycine betaine transporter Gbu but lacking BetL and OpuC; ASA6 is another double-mutant strain (SOEopuC15gbu10) possessing BetL and lacking OpuC and Gbu; finally, ASA7 is a triple mutant (SOEbetL25gbu10opuC15) having none of the three transporters (Table 1). Cultures were kept on brain heart infusion (BHI) (Difco) agar plates at 4°C. Modified (lacking choline) Pine's medium (33), a medium containing amino acids, vitamins, salts, and nucleotides, buffered by N-(2-acetamido)-2-aminoethanesulfonic acid at pH 6.75 and supplemented with 0.5% glucose, was used as the defined medium. Glycine betaine, carnitine, NaCl, and KCl were purchased from Sigma Chemical Co. (St. Louis, Mo.). [Methyl-14C]carnitine and [methyl-14C]choline were purchased from Perkin-Elmer Life Sciences, Inc. (Boston, Mass.). [Methyl-14C]glycine betaine was prepared by oxidation of [methyl-14C]choline (31).
TABLE 1.
L. monocytogenes strains used in this study
Measurement of generation time under cold stress in the presence and absence of osmolytes.
L. monocytogenes cultures were grown overnight at 30°C in BHI broth, and 1-ml aliquots were centrifuged at 11,750 × g for 10 min. The pellets were washed twice with 1-ml portions of Pine's medium and used to inoculate (0.5%) 125-ml Pyrex nephelo flasks containing Pine's medium. These flasks were grown at 30°C to cell densities of ca. 2 × 109 CFU/ml, diluted 10-fold in Pine's medium, and used to inoculate (1%) four sets of 125-ml Pyrex nephelo flasks containing 15 ml of Pine's medium. These sets were incubated with mild shaking (60 rpm) at 4°C in the absence of osmolytes or in the presence of 1 mM glycine betaine, 1 mM carnitine, or both osmolytes at a concentration of 0.5 mM each. Unstressed cultures (grown at 30°C) with or without added osmolytes served as controls. Growth was monitored with a Klett-Summerson photoelectric colorimeter with a green (no. 54) filter. Each combination of strain and osmolyte was tested in triplicate. Specific growth rate constants were calculated by plotting the natural logarithm of Klett units versus time and were converted to their respective generation timevalues.
Transport and steady-state cytoplasmic levels of glycine betaine and carnitine.
Transport of glycine betaine and carnitine was examined for the wild-type L. monocytogenes 10403S, all three double-mutant strains (ASA4, ASA5, and ASA6), and the triple-mutant strain, ASA7 at 4°C. For each strain, transport assays were done in duplicate using 100 μM [methyl-14C]glycine betaine and [methyl-14C]carnitine as described previously (23). Uptake rates were normalized to total cellular protein, which was determined using the bicinchoninic acid method (42) (Pierce Chemical, Rockford, Ill.), and are reported as nanomoles of osmolyte per minute per milligram of cellular protein.
L. monocytogenes 10403S, ASA4, ASA5, ASA6, and ASA7 were grown aerobically in modified Pine's medium, each containing 0.5 mM glycine betaine and 0.5 mM carnitine at 7°C. Unstressed cultures grown at 30°C with added osmolytes served as controls. Cultures were harvested at late log phase by centrifugation (4,080 × g, 10 min, 4°C). The pelleted cells were immediately washed with ice-cold 1% KCl solution. Cytoplasmic contents were extracted with ice-cold 7% perchloric acid as described elsewhere (41). Extracts were analyzed by natural-abundance 13C nuclear magnetic resonance (13C-NMR) as previously described (3). Total cellular protein at the time of harvest was used to normalize compatible solute concentrations.
RESULTS
Uptake of glycine betaine and carnitine by L. monocytogenes under cold stress.
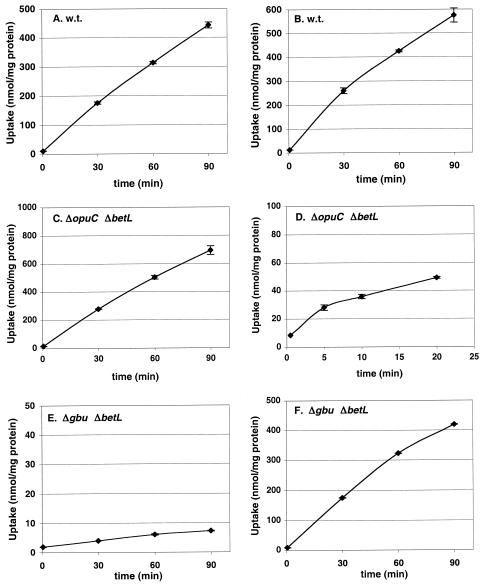
L. monocytogenes 10403S, ASA4, ASA5, ASA6, and ASA7 were grown at 4°C in modified Pine's medium. Exponentially growing cultures were centrifuged and resuspended in buffer of the same osmolality as that of the growth medium, and uptake of glycine betaine or carnitine was measured over time after addition of [methyl-14C]glycine betaine or [methyl-14C]carnitine to the cultures (Fig. 1).
FIG. 1.
Compatible solute transport activity of L. monocytogenes 10403S and mutant derivatives ASA4, ASA5, ASA6, and ASA7 at 4°C. Uptake of 100 μM [14C]glycine betaine (A, C, E, G, and I) or [14C]carnitine (B, D, F, H, and J) was measured for strain 10403S (A and B), ASA5 (C and D), ASA4 (E and F), ASA6 (G and H), or ASA7 (I and J) grown to late log phase in modified Pine's medium at 4°C. Error bars indicate the range of duplicate values. Note that the vertical scales vary.
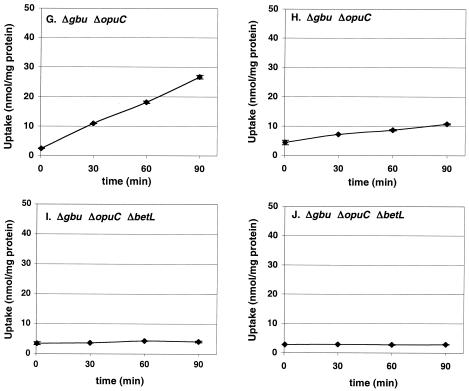
The transport of glycine betaine and carnitine for L. monocytogenes is cold activated, and the solutes are transported with comparable rates by the wild-type strain at 4°C (Fig. 1A and B). Under these conditions, the ABC transporter Gbu (Fig. 1C) represents the major pathway for glycine betaine uptake, and a minor route for carnitine uptake (Fig. 1D); carnitine transport by Gbu was weak and reached a plateau after about 30 min. The ABC transporter OpuC is the dominant porter for carnitine uptake at 4°C (Fig. 1F); the involvement of OpuC in betaine transport under chill is minor but is nonetheless present (Fig. 1E). The involvement of the sodium-betaine symporter BetL in glycine betaine transport under cold stress was found to be minor (Fig. 1G), and that of carnitine transport was even smaller (Fig. 1H); both BetL-mediated transport activities, however, were distinct from zero. Transport of either osmolyte could not be detected in the triple mutant strain L. monocytogenes ASA7 (Fig. 1I and J).
Compatible solute accumulation under chill stress.
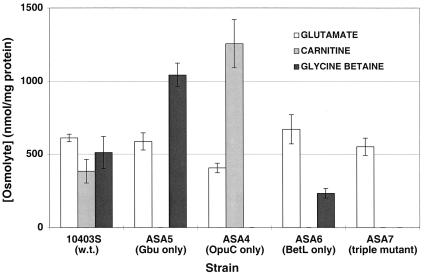
L. monocytogenes strains (10403S, ASA4, ASA5, ASA6, and ASA7) were grown in defined medium at 7°C with a 0.5 mM concentration (each) of glycine betaine and carnitine. 13C-NMR analysis was performed on perchloric acid extracts of cells from cultures harvested during exponential growth. With the exception of low levels of glutamate, no other osmolyte was present in cell extracts of any strain in the absence of chill stress (i.e., at 30°; data not shown).
When grown under chill stress, wild-type L. monocytogenes accumulated glycine betaine, carnitine, and glutamate (Fig. 2). Cell extracts of the mutant ASA5, which possesses only the Gbu transporter, contained increased levels of glycine betaine and comparable levels of glutamate relative to the wild type, but carnitine accumulation was not detected. Extracts of ASA4, which possesses only the OpuC transporter, contained increased levels of carnitine and comparable levels of glutamate relative to the wild type but no detectable glycine betaine. Extracts of the mutant ASA6, which possesses only BetL, contained wild-type levels of glutamate, a level of glycine betaine that was lower than that of the wild type, and no detectable carnitine. Glutamate was the sole detectable osmolyte in extracts of the triple mutant ASA7.
FIG. 2.
Compatible solute accumulation by L. monocytogenes 10403S and mutant L. monocytogenes ASA4, ASA5, ASA6, and ASA7 during exponential growth in modified Pine's medium at 7°C with 0.5 mM glycine betaine and carnitine. Exponentially growing cultures were harvested and washed, and cytoplasmic contents were extracted with perchloric acid. Alanine (50 mM) was added to each extract as an internal standard, and compatible solutes were quantitated by using natural-abundance 13C-NMR spectroscopy. Error bars indicate ranges of duplicate measurements.
Growth under cold stress in the presence or absence of glycine betaine and carnitine.
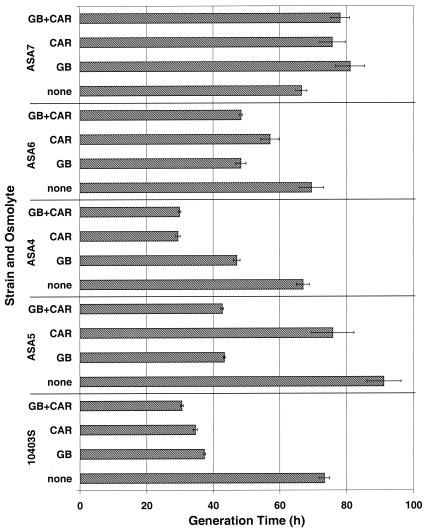
The effectiveness of each transporter in alleviating cold stress in media with different compatible solute compositions (with or without 1 mM glycine betaine or carnitine or a mixture of 0.5 mM glycine betaine and 0.5 mM carnitine) was determined by measuring the growth of the five strains in Pine's medium at 4°C (Fig. 3) and at 30°C (data not shown). Regardless of the strain or osmolyte content, cultures at 30°C grew with an average generation time of 1.33 ± 0.09 h. In the absence of compatible solutes or when all three solute transporters were deleted (strain ASA7), the generation time of cold-stressed cultures was the longest (Fig. 3).
FIG. 3.
Growth characteristics of L. monocytogenes 10403S (wild type), ASA5 (containing only Gbu), ASA4 (containing only OpuC), ASA6 (containing only BetL), and ASA7 (triple deletion) at 4°C in modified Pine's medium. Cultures were grown in BHI medium, washed, and used to inoculate (1%) modified Pine's medium. These cultures were grown to late log phase, 10-fold diluted, and used to inoculate (1%) modified Pine's medium containing 1 mM glycine betaine (GB), 1 mM carnitine (CAR), or a 0.5 mM concentration of each osmolyte (GB+CAR) or in the absence of osmolytes (none). Error bars indicate ±1 standard deviation of triplicate values.
The following differences were observed in the growth of the five strains at 4°C. Compared to the growth rate observed in the absence of compatible solutes, wild-type L. monocytogenes grew twice as fast in the presence of either solute (betaine or carnitine) in the growth medium. The mutant ASA5, which possesses only the Gbu porter, also showed a twofold reduction in generation time when glycine betaine was added. The presence of carnitine conferred markedly weaker but nonetheless measurable cryoprotection. An analogous response was exhibited by strain ASA4, which possesses only the carnitine transporter OpuC. Carnitine, the primary solute for this porter, was very effective, producing a reduction in generation time of strain ASA4 to less than half that of ASA4 cultures grown in the absence of solutes; glycine betaine was less effective, reducing the generation time by about 30%. For strain ASA6, which carries only the glycine betaine symporter BetL, the cryoprotective effect of glycine betaine was moderate and that of carnitine even weaker. Finally, the growth of the triple mutant ASA7 could not be stimulated by either compatible solute under cold stress. In fact, the inclusion of osmolytes in cultures of ASA7 under cold stress resulted in slightly lower growth rates than those from cultures of ASA7 without added osmolytes.
DISCUSSION
L. monocytogenes presents a modern challenge to the food industry worldwide that is attributable mainly to the pathogen's ability to adapt and proliferate at refrigerator temperatures. In this report we have examined the specificity for the cryoprotectants glycine betaine and carnitine of the three compatible solute transporters under low-temperature stress and compared their abilities to confer cryoprotection in environments where either, both, or neither of these solutes is present.
Under cold stress, the two L. monocytogenes multicomponent ABC transporters Gbu and OpuC were shown to play key roles in compatible solute-mediated cryoprotection. Gbu is the pathogen's major route for glycine betaine uptake. Under chill stress, strain ASA5, possessing Gbu as the sole compatible solute transporter, accumulates betaine in high concentrations and displays significant growth stimulation in the presence of the osmolyte. Gbu was also found capable of transporting carnitine, albeit much less efficiently, and cold-triggered Gbu-mediated carnitine uptake resulted in only minor restoration of growth.
OpuC is the major carnitine transporter for L. monocytogenes under both salt and chill stress (2, 10). Strain ASA4, possessing OpuC as the sole compatible solute transporter, transported and accumulated carnitine very efficiently and displayed significant growth stimulation under chill stress in the presence of the osmolyte. The data also show that OpuC is able to transport glycine betaine. Although glycine betaine transport was found to be weak, it was nonetheless measurable, and ASA4 grew approximately 30% faster under cold stress in the presence of glycine betaine than in its absence.
The osmoprotective function of BetL, the glycine betaine-Na+ symporter, has been well characterized (12, 37, 38). Data presented in this study demonstrate that BetL is also functional at low temperature. Strain ASA6 transported and accumulated betaine, most likely activated by the ambient osmotic strength and the presence of sodium ion in the growth medium. Indeed, the transport rate at 4° is actually lower than that observed at 30° (e.g., see Fig. 2G of reference 1) under essentially the same conditions, indicating that transport through BetL is not stimulated by chill, in agreement with previous work, which indicated that BetL followed normal Arrhenius kinetics in membrane vesicles (12). Despite the absence of chill activation of glycine betaine transport, strain ASA6 grew substantially faster in the presence of the solute at 4°C. A very low level of BetL-mediated carnitine transport was detected in strain ASA6, as well. Although the osmolyte was undetectable in ASA6 cell extracts, its presence in the growth medium was shown to be cryoprotective.
The triple mutant strain ASA7 showed no uptake of either glycine betaine or carnitine. The NMR spectra of ASA7 in the presence of both betaine and carnitine were devoid of both osmolytes. Finally, addition of these osmolytes individually or in combination in ASA7 cultures grown at 4°C had no cryoprotective effect. Therefore, compatible solute uptake and accumulation data and growth rate data presented here provide evidence that no other transporter for either glycine betaine or carnitine operates in L. monocytogenes 10403S at 4°C. The complete absence of compatible solute transport in strain ASA7 also demonstrates that the low rates of transport of glycine betaine by OpuC and BetL and of carnitine by BetL observed in this study (Fig. 1E, G, and H) are real and that transport proceeds through the single transporter remaining in the respective strain.
The observation that the addition of betaine or carnitine to the medium in the absence of all three transporters causes mild inhibition of growth (Fig. 3) is curious. The concentration of added compatible solutes is too low to impart significant osmotic stress to the cells. We suggest that extracellular glycine betaine and carnitine might inhibit the transport of other molecules to produce mild inhibition of growth. This inhibition would be masked in transport-competent cells by the stimulation afforded by cryoprotection. In contrast, under osmotic stress by NaCl we observed a negligible decrease in the generation time of this mutant upon addition of the same osmolytes (1).
The ABC transporters Gbu and OpuC seem to be the key mediators for the chill-activated uptake of the potent solutes glycine betaine and carnitine, respectively. In response to cold stress, both ABC transporters are capable of increasing the accumulation of their preferred compatible solute that can be transported (betaine in the case of ASA5 and carnitine in the case of ASA4) when transport of the other potent solute is impaired, a phenomenon that we have also previously observed (3). Under cold stress and in the absence of both ABC transporters, BetL is still capable of accumulating betaine in measurable levels. Finally, when L. monocytogenes is devoid of all three transporters, as is the case with the triple mutant ASA7, glutamate was the only solute accumulated.
Under salt stress (1), glutamate was accumulated, and the level of accumulation was increased 4-fold in the strain containing only BetL, 10-fold in strains containing only Gbu or OpuC, and 20-fold in the triple mutant (see Fig. 3 of reference 1). The osmotically stimulated accumulation of glutamate is well known in bacteria, particularly in gram-negative species. It is accumulated quite rapidly by biosynthesis in E. coli and is thought to function as a counterion to the transient inorganic osmolyte K+, which is accumulated via transport by the Kdp and Trk systems (5). The function of glutamate (and of K+) in gram-positive organisms is less well characterized, but it is assumed to be accumulated by biosynthesis, and it almost certainly requires a counterion, which would have to be accumulated via transport. In any case, the relative salt sensitivity of strain ASA7 indicates that the combination of glutamate and its counterion is not particularly effective in conferring osmotolerance upon L. monocytogenes (1). In contrast, the level of intracellular glutamate is not altered appreciably by chill or by deletion of any or all of the three transport systems under chill stress (Fig. 2).
The transport data and the accumulation data show that the substrate specificity of each of the three transporters is the same under osmotic stress (1) as it is under chill stress (this work): OpuC transports primarily carnitine under either stress, and Gbu transports primarily glycine betaine, but some carnitine, under either stress. BetL transports carnitine very weakly under either stress, and its ability to transport glycine betaine under chill stress is poor. Comparison of growth data for these mutants under osmotic and chill stress supports these conclusions, with two minor exceptions. Despite the slow transport and low levels of osmolyte accumulation, the presence of OpuC (strain ASA4) allows glycine betaine to confer modest cryoprotection as well as osmoprotection (1), and the presence of BetL (strain ASA6) permits weak cryoprotection by glycine betaine and even weaker cryoprotection but not osmoprotection (1) by carnitine compared to the triple mutant. Hence, all three transporters play demonstrable roles in conferring chill tolerance as well as osmotolerance.
REFERENCES
- 1.Angelidis, A. S., and G. M. Smith. 2003. Three transporters mediate uptake of glycine betaine and carnitine by Listeria monocytogenes in response to hyperosmotic stress. Appl. Environ. Microbiol. 69:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelidis, A. S., L. T. Smith, L. M. Hoffman, and G. M. Smith. 2002. Identification of OpuC as a chill-activated and osmotically activated carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2644-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelidis, A. S., L. T. Smith, and G. M. Smith. 2002. Elevated carnitine accumulation by Listeria monocytogenes impaired in glycine betaine transport is insufficient to restore wild-type cryotolerance in milk whey. Int. J. Food. Microbiol. 75:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Annous, B. A., L. A. Becker, D. O. Bayles, D. P. Labeda, and B. J. Wilkinson. 1997. Critical role of anteiso-C-15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 63:3887-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker, E. P. 1992. Cell K+ and K+ transport systems in prokaryotes, p. 205-224. In E. P. Bakker (ed.), Alkalai cation transport systems in prokaryotes. CRC Press, Boca Raton, Fla.
- 6.Bayles, D. O., B. A. Annous, and B. J. Wilkinson. 1996. Cold stress proteins induced in Listeria monocytogenes in response to temperature downshock and growth at low temperatures. Appl. Environ. Microbiol. 62:1116-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 8.Deshnium, P., Z. Gombos, Y. Nishiyama, and N. Murata. 1997. The action in vivo of glycine betaine in enhancement of tolerance of Synechococcus sp. strain PCC 7942 to low temperature. J. Bacteriol. 179:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etchegaray, J.-P., and M. Inouye. 1999. CspA, CspB, and CspG, major cold shock proteins of Escherichia coli, are induced at low temperature under conditions that completely block protein synthesis. J. Bacteriol. 181:1827-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser, K. R., D. Harvie, P. J. Coote, and C. P. O'Byrne. 2000. Identification and characterization of an ATP binding cassette L-carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4696-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 2000. Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J. Bacteriol. 182:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerhardt, P. N. M., L. T. Smith, and G. M. Smith. 1996. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicles. J. Bacteriol. 178:6105-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graumann, P., K. Schroeder, R. Schmid, and M. A. Marahiel. 1996. Cold shock stress-induced proteins in Bacillus subtilis. J. Bacteriol. 178:4611-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graumann, P., T. M. Wendrich, M. H. W. Weber, K. Schroder, and M. A. Marahiel. 1997. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol. Microbiol. 25:741-756. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi, H., Alia, L. Mustardy, P. Deshnium, M. Ida, and N. Murata. 1997. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycine betaine and enhanced tolerance to salt and cold stress. Plant J. 12:133-142. [DOI] [PubMed] [Google Scholar]
- 16.Hebraud, M., and J. Guzzo. 2000. The main cold shock protein of Listeria monocytogenes belongs to the family of ferritin-like proteins. FEMS Microbiol. Lett. 190:29-34. [DOI] [PubMed] [Google Scholar]
- 17.Holmstrom, K.-O., S. Somersalo, A. Mandal, T. E. Palva, and B. Welin. 2000. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 51:177-185. [DOI] [PubMed] [Google Scholar]
- 18.Horton, R. M., Z. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-530, 532, 534-535. [PubMed] [Google Scholar]
- 19.Jiang, W., Y. Hou, and M. Inouye. 1997. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 272:196-202. [DOI] [PubMed] [Google Scholar]
- 20.Jones, C. E., G. Shama, D. Jones, I. S. Roberts, and P. W. Andrew. 1997. Physiological and biochemical studies on psychrotolerance in Listeria monocytogenes. J. Appl. Microbiol. 83:31-35. [DOI] [PubMed] [Google Scholar]
- 21.Jones, S. L., P. Drouin, B. J. Wilkinson, and P. D. Morse. 2002. Correlation of long-range membrane order with temperature-dependent growth characteristics of parent and a cold-sensitive, branched-chain-fatty-acid-deficient mutant of Listeria monocytogenes. Arch. Microbiol. 177:217-222. [DOI] [PubMed] [Google Scholar]
- 22.Ko, R., and L. T. Smith. 1999. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl. Environ. Microbiol. 65:4040-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, S. Q., J. E. Graham, L. Bigelow, P. D. Morse, and B. J. Wilkinson. 2002. Identification of Listeria monocytogenes genes expressed in response to growth at low temperature. Appl. Environ. Microbiol. 68:1697-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastronicolis, S. K., J. B. German, N. Megoulas, E. Petrou, P. Foka, and G. M. Smith. 1998. Influence of cold shock on the fatty-acid composition of different lipid classes of the food-borne pathogen Listeria monocytogenes. Food Microbiol. 15:299-306. [Google Scholar]
- 26.Mastronicolis, S. K., J. B. German, and G. M. Smith. 1996. Diversity of the polar lipids of the food-borne pathogen Listeria monocytogenes. Lipids 31:635-640. [DOI] [PubMed] [Google Scholar]
- 27.Mastronicolis, S. K., J. B. German, and G. M. Smith. 1996. Isolation and fatty acid analysis of neutral and polar lipids of the food bacterium Listeria monocytogenes. Food Chem. 57:451-456. [Google Scholar]
- 28.Mead, P., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. Griffin, and R. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patchett, R. A., A. F. Kelly, and R. G. Kroll. 1992. Effect of sodium chloride on the intracellular solute pools of Listeria monocytogenes. Appl. Environ. Microbiol. 58:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patchett, R. A., A. F. Kelly, and R. G. Kroll. 1994. Transport of glycine betaine by Listeria monocytogenes. Arch. Microbiol. 162:205-210. [DOI] [PubMed] [Google Scholar]
- 31.Perroud, B., and D. Le Redoulier. 1985. Glycine betaine transport in E. coli: osmotic modulation. J. Bacteriol. 161:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phan-Thanh, L., and T. Gormon. 1995. Analysis of heat and cold shock proteins in Listeria by two-dimensional electrophoresis. Electrophoresis 16:444-450. [DOI] [PubMed] [Google Scholar]
- 33.Pine, L., M. J. Franzus, and G. B. Malcolm. 1986. Guanine is a growth factor for Legionella species. J. Clin. Microbiol. 23:163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puttmann, M., N. Ade, and H. Hof. 1993. Dependence of fatty acid composition of Listeria spp. on growth temperature. Res. Microbiol. 144:279-283. [DOI] [PubMed] [Google Scholar]
- 35.Raines, L. J., C. W. Moss, D. Farshtchi, and B. Pittman. 1968. Fatty acids of Listeria monocytogenes. J. Bacteriol. 96:2175-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuchat, A., K. A. Deaver, J. D. Wenger, B. D. Plikaytis, L. Mascola, R. W. Pinner, A. L. Reingold, C. V. Broome, et al. 1992. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. JAMA 267:2041-2045. [PubMed] [Google Scholar]
- 37.Sleator, R. D., C. G. M. Gahan, T. Abee, and C. Hill. 1999. Identification and disruption of betL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleator, R. D., C. G. M. Gahan, B. O'Driscoll, and C. Hill. 2000. Analysis of the role of betL in contributing to the growth and survival of Listeria monocytogenes LO28. Int. J. Food Microbiol. 60:261-268. [DOI] [PubMed] [Google Scholar]
- 39.Sleator, R. D., J. Wouters, C. G. M. Gahan, T. Abee, and C. Hill. 2001. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl. Environ. Microbiol. 67:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis SpoIIM Gene. Biochimie 74:705-711. [DOI] [PubMed] [Google Scholar]
- 41.Smith, L. T., and G. M. Smith. 1989. An osmoregulated dipeptide in stressed Rhizobium meliloti. J. Bacteriol. 171:4714-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 43.Sun, A. N., A. Camilli, and D. A. Portnoy. 1990. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verheul, A., E. Glaasker, B. Poolman, and T. Abee. 1997. Betaine and L-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J. Bacteriol. 179:6979-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verheul, A., F. M. Rombouts, R. R. Beumer, and T. Abee. 1995. An ATP-dependent L-carnitine transporter in Listeria monocytogenes Scott A is involved in osmoprotection. J. Bacteriol. 177:3205-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker, S. J., P. Archer, and J. G. Banks. 1990. Growth of Listeria monocytogenes at refrigeration temperatures. J. Appl. Bacteriol. 68:157-162. [DOI] [PubMed] [Google Scholar]
- 47.Wemekamp-Kamphuis, H. H., A. K. Karatzas, J. A. Wouters, and T. Abee. 2002. Enhanced levels of cold shock proteins in Listeria monocytogenes LO28 upon exposure to low temperature and high hydrostatic pressure. Appl. Environ. Microbiol. 68:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]