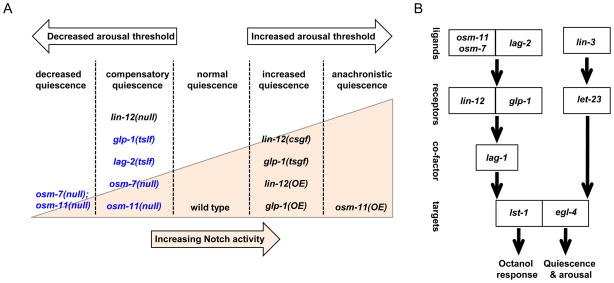
Figure 5.
Model for Notch signaling regulating multiple aspects of L4-to-adult molting quiescence. (A) Schematic model summarizing the impact of various Notch pathway genes. Gene name and type of allele is listed; Notch activity increases left to right (illustrated by peach wedge). For each genotype, the impact of altered Notch signaling on total L4-to-adult quiescence is listed above the wedge; OSM-11 co-ligand over-expression also induced anachronistic quiescence in adult animals. Decreased Notch signaling decreased arousal threshold during L4-to-adult molting lethargus while increased Notch signaling increased arousal thresholds (white arrows). Decreased glp-1 Notch receptor function, decreased DSL ligand function or loss of DOS co-ligands increased C. elegans basal locomotion activity (blue text) while loss of lin-12 Notch receptor decreased basal activity. (B) Genetic pathway illustrating relationships between C. elegans genes regulating octanol response and quiescence. egl-4 PKG also contributes, but is not required for octanol response; the role of the EGF pathway in octanol response has not been addressed. Neuronal Notch receptors act redundantly and in different populations of neurons in this behavior: lin-12 in a subset of interneurons and glp-1 in ciliated sensory neurons. Notch receptors may also act elsewhere to regulate quiescence. Activation of either EGF or Notch pathways by ligand over-expression induces anachronistic quiescence and these pathways are both required for normal L4-to-adult molting quiescence. EGF and Notch pathways may act in parallel converging on egl-4 to regulate molting quiescence (illustrated) and/or there may be functional interrelationships between these signaling pathways.