Abstract
Background
The risk of death in dialysis patients remains high, but varies significantly among patients. No prediction tool is widely used in current clinical practice. We aimed to predict long-term mortality in incident dialysis patients with easily obtainable variables.
Study Design
Prospective nationwide multicenter cohort study in the United Kingdom (UK Renal Registry); Models were developed using Cox proportional hazards.
Setting and Participants
Patients initiating hemodialysis or peritoneal dialysis between 2002 and 2004, who survived at least three months on dialysis treatment, were followed for three years. Analyses were restricted to subjects in whom information on comorbid conditions and laboratory measurements were available (n=5447). The dataset was divided into datasets for model development (n=3631, training) and validation (n=1816) by random selection.
Predictors
Basic patient characteristics, comorbidity and laboratory variables.
Outcomes
All cause mortality censored for kidney transplant, recovery of kidney function, and loss to follow-up.
Results
In the training dataset, 1078 patients (29.7%) died within the observation period. The final model of the training dataset included patient characteristics (age, race, primary kidney disease, treatment modality), comorbidities (diabetes, history of cardiovascular disease, smoking) and laboratory variables (hemoglobin, serum albumin, creatinine, calcium) and reached a C-statistic of 0.75 (95% CI, 0.73–0.77) and could accurately discriminate between patients with low (6%), intermediate (19%), high (33%) and very high (59%) mortality risk. The model was further applied to the validation dataset and achieved a C-statistic of 0.73 (95% CI, 0.71–0.76).
Limitations
Number of missing comorbidity data and lack of an external validation dataset.
Conclusions
Basic patient characteristics, comorbidity and laboratory variables can predict three-year mortality in incident dialysis patients with sufficient accuracy. Identification of subgroups of patients according to mortality risk can guide future research and subsequently target treatment decisions in individual patients.
Index Words: End stage renal disease, predictive model, mortality, hemodialysis, peritoneal dialysis
The risk of death in patients with end stage renal disease (ESRD) remains high 1, despite advances in dialysis care 2. The primary cause of death is typically cardiovascular disease (CVD), but infections and non-vascular sudden cardiac death also contribute significantly 3;4. Mortality risk varies considerably among ESRD patients, and it is dependent on preexisting conditions and comorbidities such as diabetes, CVD, anemia, impairment of bone mineral metabolism and chronic inflammation 5–7.
Several instruments have been developed to assess the burden of comorbid conditions and to predict outcome in ESRD patients 8–15, reflecting the elevated risk when compared to the general population 16. However, these approaches frequently ignore patient characteristics, such as gender, race, primary kidney disease, treatment modality or important comorbidities and none of these models is commonly used in clinical practice. Clinical experience may be sufficient to classify the patients according to their mortality risk 17, but to make risk assessment comparable and widely applicable, a more generalizable approach is needed. A predictive model that can accurately stratify patients according to their risk for mortality would be useful to assess the “case-mix” of renal centers and adjust for baseline risk in comparative studies 2;18. Such a tool could further be employed to focus future research to those patients likely to benefit from more or less intense treatment, thus providing the evidence for clinical decision making.
The purpose of the current study was to develop a model predicting all cause mortality within the first three years after starting dialysis in an unselected cohort of incident dialysis patients in the United Kingdom who were still on dialysis treatment after three months since inception. We aimed to include only readily available and easily obtainable patient characteristics, comorbidities and basic laboratory variables.
Methods
Study Participants of the United Kingdom Renal Registry
The detailed organization of the United Kingdom Renal Registry (UKRR) has been described previously 19. In brief, the UKRR is operated under the auspices of the UK Renal Association and provides independent audit and analysis of renal care in the UK. Although the UKRR now receives patient level data from all UK renal units, during the period of this study, information was prospectively collected electronically from 60 renal units in England and Wales at quarterly intervals for all patients receiving renal replacement therapy. Data arriving at the UKRR are subjected to algorithms which identify suspicious values, which are further verified and corrected if necessary by contacting the renal center.
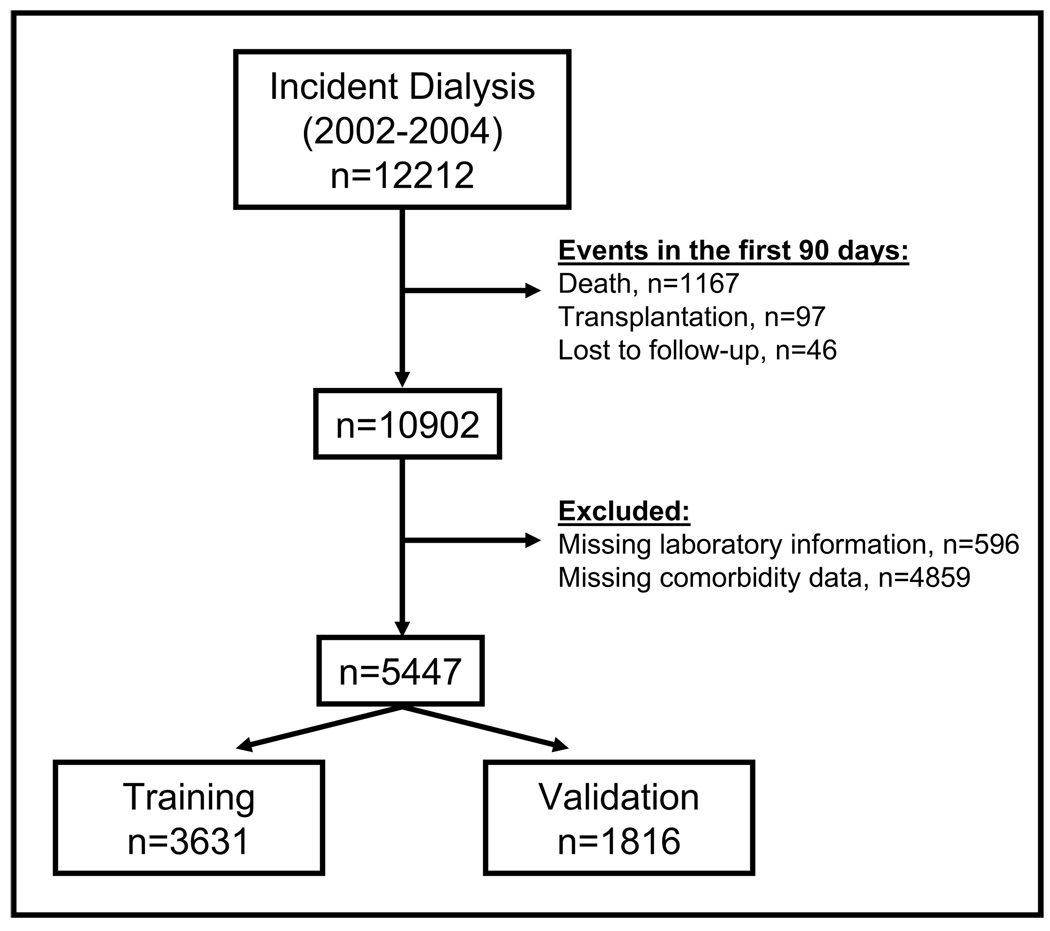
The present analysis included all adult (≥18 yrs) incident dialysis patients who started dialysis in 2002, 2003 and 2004 and who still received dialysis treatment three months after inception. Thus, we excluded patients (n=1310) who died, underwent transplant or who recovered kidney function within 90 days after initiation of dialysis (Figure 1). This reflects the standard approach to investigate “true” ESRD patients amongst all those receiving dialysis because the diagnosis of ESRD is commonly made after three months. Thus most of the patients with dialysis-dependent acute kidney injury are excluded, but unfortunately, ESRD patients who are at extremely high risk for death and die within the first three months are not considered as well. Data on study subjects were available until December 31st 2007. Patients were followed for at least three years.
Figure 1. Study participants in the UK Renal Registry.
Dataset for the analysis comprising patients in the UK Renal Registry initiating dialysis between 2002 and 2004; exclusion of patients with events within the first 90 days, or without information about comorbidities or laboratory data in the first 6 months of dialysis treatment.
Outcomes and variables of interest
The primary outcome “long-term mortality” represented all cause mortality within three years after starting dialysis treatment. Covariates of interest comprised year of dialysis initiation, age, gender, race, cause of ESRD, treatment modality (hemodialysis [HD] vs. peritoneal dialysis [PD]) at three months after dialysis inception, change of treatment modality within the first three months of treatment, and comorbidities. At initiation of dialysis treatment, the UKRR collects information about diabetes, smoking status, and history of CVD according to ischemic heart disease (angina, previous myocardial infarction, coronary artery bypass graft or angioplasty), cerebrovascular disease and peripheral vascular disease (claudication, ischemic or neuropathic ulcers, non coronary angioplasty, vascular graft or aneurysm, amputation for peripheral vascular disease) 19. Socio-economic status was assed by Townsend scores, which are based on postal codes, and range from least deprived to most deprived (score 1 to 5) 20. In addition, hemoglobin, serum albumin, calcium, phosphate, creatinine (predialysis) and ferritin were considered for the analyses. We did not include body mass index (BMI), blood pressure, cholesterol and iPTH due to missing data >20%. Anemia was defined as hemoglobin <12 g/dl in women and <13.5 g/dl in men 21.
Since we considered comorbidities and laboratory variables as being essential for the development of the prediction model, we excluded patients in whom none of the comorbidity information was available (n=4859) or in whom laboratory data of the first six months was missing (n=596) (Figure 1). Some renal centers reported comorbidity data more frequently to the UKRR than other centers 22; therefore we investigated potential differences in mortality risk between these renal units, but did not detect strong evidence for center effects (data not shown).
The purpose of our prediction model was to assess the patient’s three-year mortality risk after three months of dialysis treatment. Thus we used laboratory data that was obtained in the second quarter of dialysis treatment. If this information was missing in patients who died in the second quarter, we used laboratory measurements of the first quarter. At the start of dialysis therapy, several impairments such as anemia, metabolic acidosis, hypervolemia and hyperparathyreoidism may not be treated adequately. After three months of dialysis treatment, these variables might be better controlled; however, with the variables available in this dataset, specific impairments such as resistance to erythropoietin stimulating agents cannot be identified. The final dataset was randomly split in a training dataset (2/3 of the original cohort, n=3631) and a validation dataset (1/3 of the original cohort, n=1816) (Figure 1).
Statistical Methods
Cox proportional hazards models were used to model all cause mortality, censored for transplant, recovery of kidney function (after 90 days) and loss to follow up. Age, gender and treatment modality three months after dialysis initiation were forced in all analyses. We included the continuous outcomes ferritin, albumin and calcium in their logarithmic forms. The proportional hazards assumption was met by all variables as investigated with Schoenfeld residuals. Outliers and influence points were identified by checking deviance residuals and difference in betas (dfbetas). The performance of the models was assessed with the time-dependent C-statistic 23. Similar to the area under the receiver operator curve, it describes the probability that the model will assign the higher mortality risk to the patient who actually died as compared to the patient who remained alive or was censored. Models resulting from the training dataset were applied to the validation dataset with fixed Hazard Ratios, i.e., the variables and their exact beta-coefficients were kept as developed for the prediction model in the training dataset. To calibrate the model, mortality risk was split into quartiles and observed versus predicted risk was investigated across risk strata 24. We chose quartiles to reflect clinically useful categories of patients with low, intermediate, high and very high risk, although a sufficient number of patients and outcomes were available to investigate mortality risk across deciles, which is also commonly presented in the literature. The analyses were performed on a complete case basis. We tested the robustness of our model by (1) excluding patients who died in the second quarter and in whom laboratory variables from the first quarter were used; (2) using laboratory data from quarter one rather than from quarter two in all subjects; (3) imputing missing values by employing the Markov Chain Monte Carlo method (SAS procedure proc mi) 25. We further investigated in univariate and multivariate logistic regression models how patients with missing comorbidity and laboratory information differed from those patients in whom this information was available. All analyses were performed in SAS 9.1 (www.sas.com). The analysis of this preexisting dataset was approved by the institutional review board at Tufts Medical Center, Boston.
Results
Study population
Patient characteristics, comorbidities and laboratory data of study subjects in the training and the validation dataset are displayed in table 1. Patients were predominantly male, the median age was 64 yrs, and more than two third of the patients received hemodialysis three months after dialysis inception. A third of the patients were diabetic or reported a history of CVD. Approximately three quarters of the patients were classified as being anemic (79% in training dataset and 73% in validation dataset).
Table 1.
Characteristics of study subjects
| training dataset (n=3631) |
validation dataset (n= 1816) |
|
|---|---|---|
| age [yrs] | 64 (49; 73) | 64 (51; 74) |
| gender, male | 62.2 | 59.7 |
| BMI [kg/m2]1 | 25 (23; 29) | 25 (22; 29) |
| treatment modality2 | ||
| HD | 70.1 | 72.3 |
| PD | 29.9 | 27.7 |
| diastolic BP [mmHg]1,3 | 78±15 | 77±15 |
| systolic BP [mmHg]1,3 | 144±26 | 144±26 |
| Townsend score4 | ||
| 1 | 16.7 | 16.3 |
| 2 | 18.3 | 18.4 |
| 3 | 18.8 | 18.7 |
| 4 | 22.9 | 23.4 |
| 5 | 23.3 | 23.3 |
| race category5 | ||
| white | 83.3 | 83.9 |
| black | 5.0 | 4.3 |
| Asian | 8.4 | 9.2 |
| Chinese | 0.7 | 0.6 |
| other | 2.6 | 2.1 |
| cause of ESRD | ||
| diabetes | 21.8 | 19.8 |
| glomerulonephritis | 10.5 | 12.1 |
| polycystic kidney disease | 7.1 | 7.1 |
| pyelonephritis | 8.5 | 8.1 |
| renovascular disease | 15.6 | 16.2 |
| other | 15.1 | 14.9 |
| uncertain | 21.4 | 21.8 |
| comorbidity | ||
| diabetes6 | 29.1 | 27.5 |
| CVD7 | 32.4 | 34.6 |
| smoking | 17.2 | 16.1 |
| laboratory measurements | ||
| albumin [g/dL] | 3.5 (3.2; 3.9) | 3.5 (3.1; 3.9) |
| calcium [mg/dL] | 9.6±0.9 | 9.6±0.9 |
| phosphate [mg/dL] | 5.1±1.7 | 5.0±1.6 |
| iPTH [pg/mL]1 | 144 (60; 285) | 148 (62; 303) |
| cholesterol [mg/dL]1 | 170 (143; 201) | 174 (143; 205) |
| ferritin [ng/mL] | 284 (164; 458) | 292 (171; 472) |
| hemoglobin [g/dl] | 11.3±1.8 | 11.3±1.8 |
| SCr [mg/dL]3 | 7.2 (5.6; 9.2) | 7.2 (5.5; 9.1) |
Categorical data are given as percentage; continuous Data are means ± standard deviation or medians (25th; 75th percentile)
abbreviations: BMI, body mass index; PD, peritoneal dialysis; HD, hemodialysis; ESRD, end stage renal disease; CVD, cardiovascular disease; iPTH, intact parathyroid hormone; BP, blood pressure; SCr, serum creatinine
conversion factors for units: serum creatinine in mg/dL to mol/L, ×88.4; cholesterol in mg/dL to mmol/L ×0.02586, albumin in g/dL to g/L ×10; calcium in mg/dL to mmol/L ×0.2495; phosphorus in mg/dL to mmol/L ×0.3229; no conversion necessary for ferritin in ng/mL and ug/L, and iPTH in pg/mL and ng/L
>20% missing data
Treatment modality after 3 months of dialysis treatment
Predialysis measurements
Socioeconomic status; least deprived (score 1), most deprived (score 5)
Self reported, patients of South Asian origin (i.e. excludes Chinese patients) are classified as “Asian”
Including diabetes as non primary cause of ESRD
Cardiovascular disease assessed according to a questionnaire about ischemic heart disease, cerebrovascular disease and peripheral vascular disease
Outcomes
In the training dataset, a total of 1078 patients (29.7%) died within the observation period from day 90 until three years (Table 2), while 563 patients (15.5%) underwent transplant. Half of the patients were alive and still on dialysis after three years. Only a few patients were either lost to follow-up or recovered kidney function (approximately 1.5% each). Similar results were observed in the validation dataset.
Table 2.
Outcomes within 3 years of dialysis initiation
| training dataset (n=3631) |
validation dataset (n=1816) |
|
|---|---|---|
| death | ||
| Percentage reaching outcome | 29.7 | 30.7 |
| time to outcome [days] | 508 (286; 772) | 478 (258; 777) |
| transplant | ||
| Percentage reaching outcome | 15.5 | 13.6 |
| time to outcome [days] | 553 (345; 816) | 501 (313; 721) |
| recovery of kidney function | ||
| Percentage reaching outcome | 1.5 | 1.5 |
| time to outcome [days] | 278 (165; 495) | 310 (129; 466) |
| loss to follow-up | ||
| Percentage reaching outcome | 1.4 | 1.3 |
| time to outcome [days] | 556 (393; 826) | 459 (263; 730) |
Times to outcome are medians (interquartile range).
Predictors of Mortality
When only patient characteristics were included in the multivariate model (Table 3, model 1), older age, white race, diabetes as cause of ESRD and HD treatment (versus PD) were independently associated with an increased risk for all cause mortality. In this analysis, a change from HD to PD within the first three months of renal replacement therapy was associated with a lower mortality risk as compared to a change from PD to HD. However, this association did not achieve formal significance level (p=0.07) and was further diluted in subsequent modeling. In a second step (model 2), the comorbidities diabetes, history of CVD and smoking status were added and also independently associated with worse outcome. The interaction term built by diabetes and CVD emerged as significant, indicating a different effect of diabetes on mortality depending on whether or not the patient had a history of CVD. Finally (model 3), the laboratory measures of hemoglobin, serum albumin, creatinine and calcium appeared as predictors of mortality, after accounting for phosphate and ferritin.
Table 3.
Mortality within 3 years of dialysis initiation, training dataset
| model 11 (n=3232) |
model 21 (n=2815) |
model 32 (n=2388) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | Wald χ2 | HR (95% CI) | Wald χ2 | HR (95% CI) | Wald χ2 | ||||
| Predictors of Mortality | |||||||||
| age | 1.04 (1.03–1.05) | 177.6 | 1.04 (1.03–1.05) | 152.5 | 1.04 (1.03–1.04) | 99.3 | |||
| gender, male | 1.03 (0.89–1.18) | 0.1 | 0.93 (0.80–1.08) | 0.9 | 0.95 (0.80–1.12) | 0.4 | |||
| treatment modality3 | 1.25 (1.05–1.47) | 6.5 | 1.19 (1.00–1.43) | 3.1 | 1.32 (1.09–1.60) | 8.3 | |||
| race | 18.8 | 14.5 | 9.9 | ||||||
| black vs. white | 0.47 (0.30–0.71) | 0.49 (0.31–0.78) | 0.50 (0.29–0.88) | ||||||
| Asiana vs. white | 0.68 (0.51–0.90) | 0.73 (0.54–0.99) | 0.72 (0.50–1.03) | ||||||
| Chinese vs. white | 0.72 (0.27–1.93) | 0.39 (0.10–1.57) | 0.45 (0.11–1.80) | ||||||
| other vs. white | 1.01 (0.67–1.53) | 1.10 (0.69–1.76) | 0.80 (0.36–1.80) | ||||||
| cause of ESRD | 75.7 | 66.9 | 50.3 | ||||||
| GN vs.DM | 0.36 (0.25–0.50) | 0.50 (0.32–0.77) | 0.48 (0.30–0.76) | ||||||
| PKD vs. DM | 0.31 (0.20–0.48) | 0.43 (0.25–0.72) | 0.54 (0.31–0.96) | ||||||
| pyelonephritis vs. DM | 0.59 (0.45–0.79) | 0.78 (0.54–1.12) | 0.94 (0.64–1.39) | ||||||
| RVD vs. DM | 0.80 (0.65–0.98) | 0.99 (0.74–1.34) | 1.12 (0.81–1.54) | ||||||
| other vs. DM | 1.07 (0.87–1.31) | 1.62 (1.18–2.21) | 1.72 (1.22–2.43) | ||||||
| uncertain vs. DM | 0.72 (0.59–0.87) | 0.98 (0.72–1.32) | 1.18 (0.85–1.65) | ||||||
| DM4 | -- | -- | -- | 1.58 (1.18–2.11) | 9.2 | 1.77 (1.28–2.44) | 11.8 | ||
| CVD5 | -- | -- | -- | 1.55 (1.29–1.86) | 21.8 | 1.56 (1.28–1.90) | 19.8 | ||
| diabetes × CVD6 | -- | -- | -- | 0.67 (0.50–0.91) | 6.8 | 0.61 (0.44–0.84) | 9.0 | ||
| smoking | -- | -- | -- | 1.41 (1.18–1.69) | 14.0 | 1.38 (1.14–1.68) | 11.1 | ||
| hemoglobin [per 1-g/dl] | -- | -- | -- | -- | -- | -- | 0.93 (0.89–0.98) | 8.2 | |
| albumin [per log g/L] | -- | -- | -- | -- | -- | -- | 0.14 (0.09–0.21) | 95.9 | |
| SCr [per 1-mg/dL/] | -- | -- | -- | -- | -- | -- | 0.96 (0.93–0.99) | 4.1 | |
| calcium [per log mg/dL] | -- | -- | -- | -- | -- | -- | 2.72 (1.13–6.58) | 4.9 | |
| Model Performance | |||||||||
| C-statistic (95% CI) | C-statistic (95% CI) | C-statistic (95% CI) | |||||||
| Concordance | 0.69 (0.67–0.71) | 0.70 (0.68–0.72) | 0.75 (0.73–0.77) | ||||||
Variables age, gender and treatment modality forced in all multivariate Cox proportional hazards models;
abbreviations: HR, hazard ratio; CI, confidence interval; ESRD, end stage renal disease; GN, glomerulonephritis; PKD, polycystic kidney disease; RVD, renovascular disease; CVD, cardiovascular disease; DM, diabetes mellitus; SCr, serum creatinine
Adjusted for start-year of dialysis, Townsend score, change in treatment modality within the first 3 months of dialysis treatment
Adjusted for start-year of dialysis, Townsend score, change in treatment modality within the first 3 months of dialysis treatment, phosphate, ferritin (log)
Treatment modality (hemodialysis vs. peritoneal dialysis) after 3 months of dialysis treatment
Including DM as non primary cause of ESRD
Cardiovascular disease assessed according to a questionnaire about ischemic heart disease, cerebrovascular disease and peripheral vascular disease
Interaction term of diabetes and CVD
“Asian” defined as patients of South Asian origin (i.e. excludes Chinese patients)
The accuracy of the Cox proportional hazards models was assessed by the C-statistic (Table 3). The model with basic patient characteristics already achieved considerable accuracy as indicated by a C-statistic of 0.69 (95% confidence interval [CI], 0.67–0.71; model 1), which further increased by adding comorbidity data and laboratory measurements (C-statistic, 0.75; 95% CI, 0.73–0.77]), mainly due to the latter.
To check the robustness of the model, we performed a number of sensitivity analyses. First, we excluded patients who died in their second quarter of dialysis treatment and in whom laboratory measurements from the first quarter were used rather than from quarter two. The variables of the final model remained unchanged, and the hazard ratios were similar, as was the C-statistic (0.74; 95% CI, 0.72–0.77). Second, when we used laboratory measurements from quarter one in all patients of the training dataset, the model achieved a C-statistic of 0.72 (95% CI, 0.69–0.75) with all variables of the final model except race and hemoglobin. Finally, since the described analyses were performed on the basis of complete cases, we imputed all missing data. The hazard ratios of the complete case model were within the 95% confidence limits of the hazard ratios derived from the imputation.
As mentioned, we excluded approximately 50% of the original cohort because of missing comorbidity and laboratory data. These patients differed from patients in whom comorbidity information was available; they had a higher three year mortality (38.6% vs. 29.7%), were older, were more likely to be of white race and had an uncertain diagnosis of primary kidney disease (all p<0.001). In multivariate logistic regression modeling aiming to identify risk factors for comorbidity and laboratory data being missing, only race, primary kidney disease and PD treatment emerged as independent predictors (detailed data not shown). We did not impute missing comorbidity data on these patients. In order to confirm the validity of our results in this population, we applied the multivariate prediction model with fixed hazard ratios to these patients including only basic patient characteristics (model 1) which were available and achieved similar discrimination (C-statistic 0.68; 95% CI, 0.65–0.71).
Calibration of the Model
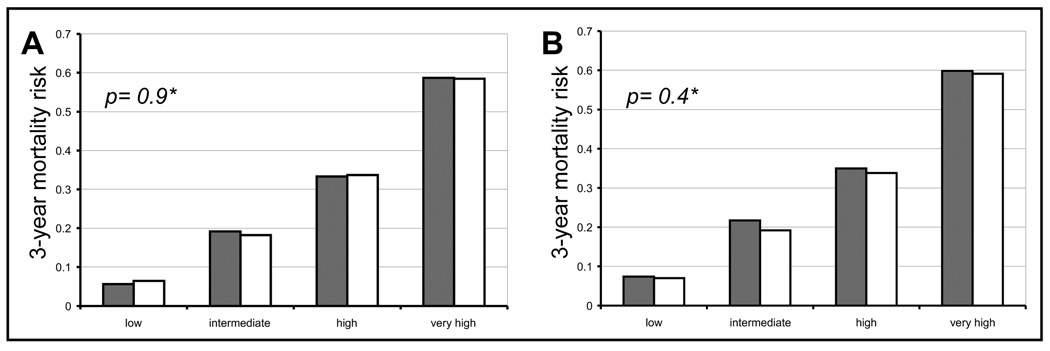
We categorized patients in the training dataset according to their three-year mortality risk into quartiles (Figure 2, panel A). The model could accurately discriminate between patients with a low (6%), intermediate (19%), high (33%) and very high (59%) mortality risk. No significant difference between observed and predicted mortality risk was found (p=0.9).
Figure 2. Three year mortality according to risk strata, calibration of the model.
Observed (grey columns) and predicted (white columns) mortality risk within 3 years after starting dialysis treatment in patients in the UK Renal Registry according to risk strata derived from the final prediction model; panel A: training dataset, panel B: validation dataset; * p-value between observed and predicted mortality risk.
Validation of the model
When we applied the final model with fixed hazard ratios to the validation dataset, the resulting C-statistic was 0.73 (95% CI, 0.7– 0.76). Observed and predicted mortality risk in the validation dataset did not significantly differ (p=0.4) across risk strata: 7%, 20%, 34%, and 59% in low, intermediate, high, and very high mortality risk, respectively (Figure 2, panel B).
Discussion
In the UK Renal Registry database we developed a model that predicted three-year mortality in patients who survived up to three months after dialysis inception and were still on dialysis treatment. The model included exclusively easily obtainable and routinely collected patient characteristics and laboratory variables. It achieved sufficient accuracy and was able to discriminate accurately across risk strata.
It is a particular strength of this model that the underlying data were prospectively collected on an incident cohort without any exclusion criteria. Data in the UKRR are considered of good quality 26 and are representative for the UK and likely even for larger parts of Europe19. We also included only variables which are routinely captured in the UK and worldwide, making the model widely applicable to a variety of settings. Furthermore, we judged the outcome three year all cause mortality – a very heterogeneous outcome with a variety of underlying causes – as more important than short term mortality 9;12;15 or events with less heterogeneity (e.g., CVD events 8), although these are frequently easier to predict.
There are only a few reports predicting long-term mortality in dialysis patients that employed Cox models and provided data on model performance. Their results are in line with our findings that patient characteristics, comorbidity data and basic laboratory variables are important to predict mortality in long-term dialysis patients. Geddes et al. retrospectively collected data about dialysis patients who died in 1997 from voluntarily participating renal centers throughout Europe 10. Their analyses were thus based on patients who differed in their time to death. Hence, patients who survived on dialysis or underwent transplant in the same observation period were not included. Their model was further validated prospectively in patients starting dialysis in the same renal centers and who were followed up to five years. Unfortunately, the measures of accuracy provided, negative and positive predictive values, ignore information on time to the event. The recently published index developed by Liu et al. 11 used data from the United States Renal Data System (USRDS) to primarily assess the prognostic value of comorbidities on mortality in incident dialysis patients who survived the first 9 months on treatment. Patients undergoing transplant were excluded and laboratory variables were not considered. Miskulin et al. described how a variety of comorbid conditions impact on three-year mortality in prevalent dialysis patients of the US population in the Dialysis Outcome and Practice Patterns Study (DOPPS) 14. Their final model reached a C-statistic of 0.73 (95% CI, 0.72–0.74) and included patient characteristics, basic laboratory variables, comorbidities (17 variables) and physical impairments (3 variables). However, this model lacked routinely collected variables and could not be electronically implemented into practice.
Although our model – as well as those from other groups – achieved considerable accuracy and discrimination to classify patients according to their mortality risk, we suggest that the results of this risk assessment should not (yet) be applied to individual patients in current clinical practice. Therefore we refrained from transforming the model into an easy-to-use score at this stage. While it seems intuitive that the model may provide clinically useful information regarding a patient being classified as “low risk” (with a 6% three-year mortality risk) as compared to a “very high risk patient” (with a mortality risk up to 60%), any modulation of therapy and treatment decisions based on this classification lack prospective clinical evidence. Once the validity of our prediction model was prospectively proven to have an impact on modulating therapy and clinical outcomes, it can be implemented in daily practice. Such a “risk-stratification-tool” could either be employed as a clinical score or embedded in computer based management systems.
In our model we show that basic patient characteristics and laboratory markers are sufficient to classify patients according to their mortality risk. These variables are not novel or surprising, and in the last decade a variety of more specific factors, for example cardiac function or biomarkers, such as CRP, adiponectin or NT-proBNP (N-terminal pro-brain natriuretic peptide) 27–31, have been found to be associated with mortality in ESRD patients. The challenge would be to test the markers regarding their incremental value on the performance of our model, since the model already achieved good accuracy and discrimination. In particular, it may be relevant to examine how many patients get correctly (re-)classified by including versus ignoring the additional variables 32.
Furthermore, the model can form the basis for risk-stratified analyses of randomized controlled trials (RCTs). Summary results of RCTs may reflect the treatment effect of a relatively small subgroup of influential patients, and do not accurately describe the treatment effect of the typical patient enrolled in the trial. While conventional subgroup analyses have important limitations, risk-stratified analyses can frequently identify patients who particularly benefit from a given treatment if there is considerable variation of baseline risk33. Since the latter is certainly the case in the setting of dialysis, it is conceivable that an analysis according to the patient’s mortality risk would provide clinical useful information.
Finally, the prediction model can be useful to evaluate the composition of patients treated in a given center. Demographic and comorbidity features vary between facilities within certain areas, which impact on mortality; this scenario is best described as variability in “case-mix” 2. Financial incentives to improve quality of care are increasingly considered in many health care systems throughout the world 34. In the US, dialysis centers are evaluated by performance and reimbursement is dependent on the “success” of the treatment of each center (“pay for performance”) 35. A similar scenario is in discussion in the UK between the NHS Information Centre, the NHS Healthcare Quality Improvement Programme (HQIP) and the renal community. We hypothesize that our prognostic model could potentially be used to create a “standardized mortality ratio” for UK renal units, making data between centers more comparable to patients, health care providers, insurance companies and researchers.
We are aware of several limitations of the current study. First, a priori to the modeling process, we excluded a large number of patients in whom comorbidity and laboratory data were not available. Although some of these variables were not missing completely at random, we could not find center effects for missingness. Our basic model also validated well in patients in whom these important data were missing. This finding suggests that the differences in mortality rate may in fact be explained by the covariates in the model. We therefore argue that the final model could be generalizable to the cohort with missing comorbidity and laboratory data. As the amount of missing data decreases prospectively in the UK, it is possible that the performance of our model may be further improved with the addition of these variables. Second, certain parameters that may have improved the performance of our model were not available, such as CRP-level, information on dialysis access, dialysis dose or residual renal function; or due to a large number of missing data (BMI, blood pressure, iPTH, cholesterol). Third, although we tested the robustness of our model extensively and internally validated it in an independent subset of patients of the UKRR, the results of our analyses need to be further externally validated in independent datasets. For example, comorbidities such as CVD were listed as composites in the UKRR, but may be available in a more detailed form in alternative datasets. It would be interesting, how the combination and importance of variables holds in other settings. Finally, we employed Cox proportional hazards models to predict all cause mortality. Although the advantages of considering time to the event and including information on patients who underwent transplant, recovered kidney function or were lost to follow-up as censored events clearly outweigh the disadvantages of ignoring these patients, this approach assumes that the patients who died post transplant had a similar risk of death as those not transplanted, and may result in errors in model calibration. We achieved excellent calibration but different censoring patterns in other dialysis populations may affect the performance of the model.
Our model suggests that a limited number of basic patient characteristics and easily obtainable and commonly measured variables are sufficient to predict with good accuracy three-year mortality in patients who survived the first three months of dialysis treatment. We would propose that novel biomarkers or other associations predicting mortality in long-term dialysis patients should be added to and tested against our model to determine their incremental value. Identification of subgroups of patients according to mortality risk can guide future research that may be able to target treatment decisions in individual patients.
Acknowledgements
We thank the patients and the clinicians in the renal units who contributed data to the UK Renal Registry as well as we thank Julie Gilg at the UK Renal Registry for administrative support. We thank Hocine Tighiouart for providing the SAS macros for calculation of the C-statistic and model calibration in Cox proportional hazards models. We also thank Andrew S. Levey and Bertram L. Kasiske for helpful discussions and critical revision of the manuscript.
Support: Dr Wagner receives funding from the fellowship training program of the National Kidney Foundation Center for Clinical Practice Guideline Development and Implementation at Tufts Medical Center. Dr Kent is supported by a grant of the National Institute of Health (NIH/NCRR 1UL1 RR025752). Dr Tangri is supported by the KRESCENT postdoctoral fellowship award, a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research and the Canadian Society of Nephrology. The UK Renal Registry is funded by an annual capitation charge to UK renal units and is independent of industry and government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
REFERENCES
- 1.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 2.Goodkin DA, Young EW, Kurokawa K, et al. Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis. 2004;44:16–21. doi: 10.1053/j.ajkd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 3.McDonald SP, Marshall MR, Johnson DW, et al. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20:155–163. doi: 10.1681/ASN.2007111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarnak MJ. Cardiovascular complications in chronic kidney disease. Am J Kidney Dis. 2003;41:11–17. doi: 10.1016/s0272-6386(03)00372-x. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 6.Menon V, Wang X, Greene T, et al. Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis. 2003;42:44–52. doi: 10.1016/s0272-6386(03)00407-4. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DE, Tighiouart H, Vlagopoulos PT, et al. Effects of anemia and left ventricular hypertrophy on cardiovascular disease in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16:1803–1810. doi: 10.1681/ASN.2004070597. [DOI] [PubMed] [Google Scholar]
- 8.Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 9.Barrett BJ, Parfrey PS, Morgan J, et al. Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis. 1997;29:214–222. doi: 10.1016/s0272-6386(97)90032-9. [DOI] [PubMed] [Google Scholar]
- 10.Geddes CC, van Dijk PC, McArthur S, et al. The ERA-EDTA cohort study--comparison of methods to predict survival on renal replacement therapy. Nephrol Dial Transplant. 2006;21:945–956. doi: 10.1093/ndt/gfi326. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Huang Z, Gilbertson DT, et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010;77:141–151. doi: 10.1038/ki.2009.413. [DOI] [PubMed] [Google Scholar]
- 12.Mauri JM, Cleries M, Vela E. Design and validation of a model to predict early mortality in haemodialysis patients. Nephrol Dial Transplant. 2008;23:1690–1696. doi: 10.1093/ndt/gfm728. [DOI] [PubMed] [Google Scholar]
- 13.Merkus MP, Jager KJ, Dekker FW, et al. Predictors of poor outcome in chronic dialysis patients: The Netherlands Cooperative Study on the Adequacy of Dialysis. The NECOSAD Study Group. Am J Kidney Dis. 2000;35:69–79. doi: 10.1016/s0272-6386(00)70304-0. [DOI] [PubMed] [Google Scholar]
- 14.Miskulin D, Bragg-Gresham J, Gillespie BW, et al. Key comorbid conditions that are predictive of survival among hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1818–1826. doi: 10.2215/CJN.00640109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miskulin DC, Martin AA, Brown R, et al. Predicting 1 year mortality in an outpatient haemodialysis population: a comparison of comorbidity instruments. Nephrol Dial Transplant. 2004;19:413–420. doi: 10.1093/ndt/gfg571. [DOI] [PubMed] [Google Scholar]
- 16.Shah DS, Polkinghorne KR, Pellicano R, et al. Are traditional risk factors valid for assessing cardiovascular risk in end-stage renal failure patients? Nephrology (Carlton) 2008;13:667–671. doi: 10.1111/j.1440-1797.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 17.Moss AH, Ganjoo J, Sharma S, et al. Utility of the "surprise" question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379–1384. doi: 10.2215/CJN.00940208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miskulin DC, Meyer KB, Martin AA, et al. Comorbidity and its change predict survival in incident dialysis patients. Am J Kidney Dis. 2003;41:149–161. doi: 10.1053/ajkd.2003.50034. [DOI] [PubMed] [Google Scholar]
- 19.Ansell D. UK Renal Registry 11th Annual Report (December 2008) Nephron Clin Pract. 2009;111 Suppl 1 doi: 10.1159/000209991. [DOI] [PubMed] [Google Scholar]
- 20.Udayaraj UP, Ben-Shlomo Y, Roderick P, et al. Ethnicity, socioeconomic status, and attainment of clinical practice guideline standards in dialysis patients in the United kingdom. Clin J Am Soc Nephrol. 2009;4:979–987. doi: 10.2215/CJN.06311208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Kidney Foudnation. KDOQI: II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47:S16–S85. doi: 10.1053/j.ajkd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Udayaraj U, Tomson CR, Gilg J, et al. UK Renal Registry 11th Annual Report (December 2008): Chapter 6 Comorbidities and current smoking status amongst patients starting renal replacement therapy in England, Wales and Northern Ireland: national and centre-specific analyses. Nephron Clin Pract. 2009;111 Suppl 1:c97–c111. doi: 10.1159/000209995. [DOI] [PubMed] [Google Scholar]
- 23.Mandel M, Galai N, Simchen E. Evaluating survival model performance: a graphical approach. Stat Med. 2005;24:1933–1945. doi: 10.1002/sim.2089. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, et al. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21:45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 25.Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman and Hall; 1997. [Google Scholar]
- 26.Tangri N, Ansell D, Naimark D. Predicting technique survival in peritoneal dialysis patients: comparing artificial neural networks and logistic regression. Nephrol Dial Transplant. 2008;23:2972–2981. doi: 10.1093/ndt/gfn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drechsler C, Krane V, Winkler K, et al. Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney Int. 2009;76:567–575. doi: 10.1038/ki.2009.200. [DOI] [PubMed] [Google Scholar]
- 28.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 29.Svensson M, Gorst-Rasmussen A, Schmidt EB, et al. NT-pro-BNP is an independent predictor of mortality in patients with end-stage renal disease. Clin Nephrol. 2009;71:380–386. doi: 10.5414/cnp71380. [DOI] [PubMed] [Google Scholar]
- 30.Yamada S, Ishii H, Takahashi H, et al. Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin J Am Soc Nephrol. 2010;5:1793–1798. doi: 10.2215/CJN.00050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 33.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 34.McDonald R, Harrison S, Checkland K, et al. Impact of financial incentives on clinical autonomy and internal motivation in primary care: ethnographic study. BMJ. 2007;334:1357. doi: 10.1136/bmj.39238.890810.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal MB, Frank RG, Li Z, et al. Early experience with pay-for-performance: from concept to practice. JAMA. 2005;294:1788–1793. doi: 10.1001/jama.294.14.1788. [DOI] [PubMed] [Google Scholar]