Abstract
Purpose of review
The aim of this review is to bring to attention the most recent advances made in understanding the role of complement components in both innate and adaptive immune responses in solid organ transplantation with emphasis on the kidney.
Recent findings
Alongside recent findings related to the role of anaphylatoxins in modulating adaptive immune responses, there has been a genomic study to assess the expression of inflammatory markers in kidney transplantation, showing significant involvement of some complement molecules in predicting graft function. Modulators of complement pathway activity such as Decay Accelerating factor (CD55) and CD59 have also been shown to have a role in graft rejection. Potential new therapeutic targets related to complement proteins are being investigated.
Summary
The mechanism of rejection in solid organ transplantation is influenced by the initial inflammatory response and subsequent adaptive alloimmune response, both of which have been shown to be affected by various complement components. Due to limitations of existing treatments, new approaches are needed to better control these responses to improve graft survival. Built on an expanding knowledge of complement involvement, targeted blocking of the effector complement molecules and modulating the expression of complement inhibitors has suggested potentially useful approaches for reducing the effect of inflammatory damage from cold ischaemia as well as reducing the activation of the adaptive immune system related to complement.
Keywords: Complement, transplantation, rejection
Introduction
There is a wide gap between the demand for organs for transplantation and the rate of organ donation, with demand growing at a much faster rate than donation. This makes preservation and maximal usage of the donated organs a major priority.
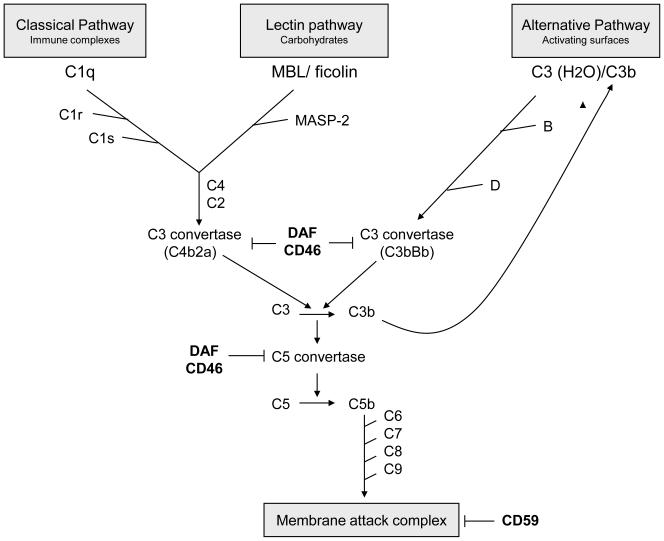
Complement proteins have been shown to play a significant part in organ damage following transplantation both in the process of ischaemia reperfusion and in modulating the activation of the adaptive immune response. There has been increasing interest in understanding the role of various complement components in this process and there is good foundation to hope that modulating the function of some of these molecules can reduce transplant organ damage and increase the organ lifespan. In this review we have discussed some of the most recent advances made in understanding the role of complement in transplantation. A map of the complement system is shown in Figure 1.
Figure 1.
Overview of the complement cascade. Activation of all three complement pathways results in cleavage of C3, leading to formation of C3a and C5a and ultimately membrane attack complex. Decay accelerating factor (DAF), CD46 and CD59 are complement regulatory molecules.
Update on the role of complement in innate-immune mediated injury
A role for complement in the pathogenesis of ischaemia-reperfusion injury has been established in a number of organ models, including the kidney. The mechanism of renal reperfusion injury depends on the generation of C5a and C5b-9 [1], both of which have direct toxicity on the renal tubules contributing to acute tubular necrosis and apoptosis, and leading to post-ischaemic acute renal failure and tissue fibrosis. In turn, the generation of these terminal pathway components depends on intra-renal synthesis of C3 and availability of other complement components that are essential for complement activation [2]. Moreover, the level of expression of C3 in the donor organ is strongly dependent on the cold ischaemic time [3]. Until recently, however, little was known about the functional relevance of these findings to man.
It is well established that living donor kidney transplants are likely to survive longer than deceased donors. Several studies indicated that prolonged cold ischaemia time in cadaveric kidney transplantation is associated with increased incidence of delayed graft function [4,5] which in turn reduces long term graft survival [6]. To understand the mechanism for the difference in survival between living and deceased donor organs, Naesens et al [7] examined the whole genome expression profile of 53 human kidney allografts using microarrays. They found a significant difference in the expression of complement genes C1 (including C1q, C1r and C1s), C2, C3, C4, C6 and complement factor B between the live and deceased donor kidneys before implantation. They also demonstrated that the complement gene expression from biopsy samples at the time of implantation had significant correlation with both early and late graft survival. Other than explaining a potential mechanism for solid organ dysfunction resulting from prolonged cold ischaemia, this study suggests that increase in the expression of complement components could be related to immune activation responsible for future rejection episodes, which have a higher frequency in recipients of deceased donor organs (see below). Based on these findings, it is plausible that therapeutic interventions targeting complement components could be effective for reducing organ damage at the time of organ recovery, transfer and after transplantation.
Update on the role of complement in cell mediated immunity
A number of murine studies in the past few years have investigated the interaction between the complement components and activation of the adaptive immune response [8,9]. These have highlighted a potential role for C3a receptor (C3aR) and C5aR signalling in antigen presenting cells (APC) and T cells. In turn, the local generation of C3a and C5a is dependent on the APC-autonomous production of complement components C3 and C5. These are secreted by dendritic cells (DC) and macrophages and contribute to immune activation. We refer the reader to an extensive review of this subject in a previous issue of this journal [10].
Additional to the demonstration that locally produced C3a and C5a interact with their receptors on antigen presenting cells and T cells and act via the expression of costimulatory molecules CD28 and CD40L to induce T cell proliferation and differentiation [11], there has been further investigation into the role of complement components on T cell activation and function. Peng et al [12] found that C5a-C5aR interaction had a direct effect on DC function in stimulation of allospecific T cells. They observed that DCs from C5aR deficient mice, or wild type DCs treated with C5aR antagonist, produce increased amounts of IL-10 and reduced amounts of IL-12p70 following LPS stimulation and have less MHC class II and B7.2 expression which leads to their reduced capacity to stimulate allospecific T cells. More recently Weaver et al [13] have shown that development of regulatory T cells and Th17 cells are affected by signalling of C5aR in DCs. They demonstrated that in the absence of C5aR, there is an increase in production of TGF-beta which directs the CD4+ T cells to differentiate to Foxp3+ Tregs. Kemper et al [14] had previously shown the effect of the regulatory complement component, CD46, in the development of Tregs. They have more recently demonstrated that this population of Tregs in contrast to other IL-10 secreting Tregs, support B cell function in that co-culturing of these Tregs with naïve B cells, promoted Ab response [15]. The effect of complement on adaptive immunity is not limited to DCs; in a very interesting study, Raedler et al [16] have shown that following stimulation with proinflammatory cytokines, endothelial cells activate the alternative pathway of complement leading to production of C5a. They have identified that this locally produced C5a results in proliferation and expansion of CD8+ T-cells, hence having a potential role in T-cell mediated injury.
A recent finding related to the communication between complement and adaptive immunity is identification of the role of C1q in T cell activation. Csomor et al [17] previously found that C1q can induce the maturation of monocytic DCs and increase their secretion of IL-12 and TNF-α as well as enhancing their T-cell stimulating property. The T cells that were stimulated by C1q-treated DCs displayed increased INF-γ production indicative of a Th-1 response. More recently, Baruah et al [18] demonstrated that C1q is an essential component for generation of a Th1-type response, which includes T cell proliferation and production of IFN-γ. They noticed that DC from C1q deficient mice could not stimulate the proliferation of MHC class II restricted antigen specific T cells or support the differentiation of these cells to effector Th1 cells. These studies point to the important influence of the complement component C1q as part of the innate immune system in modulating the adaptive immune response. The increase in expression of C1q in transplanted kidney with increased cold ischaemia time shown by Naesen et al [7] could perhaps result in increased T cell response and potentially higher chance of rejection, by direct action of C1q on APC function. Although C1q, as an essential trigger of the classical pathway, could lead to enhanced functions of complement through cleavage of C3, classical pathway activation appears to be non contributory to cell mediated rejection, at least in mice [19].
A detailed study of the association between complement C3 and its role in DC proliferation and adaptive immune response has been made possible by identifying an individual with C3 deficiency [20]. Total absence of C3 is a rare condition, which is associated with recurrent bacterial infections. In this case, the authors found the patient with C3 deficiency to have impairment in maturation of monocytes to DCs and to lack memory B cells and have poor antibody production following vaccination. In addition, CD4+ T-cells from the patient were not able to induce Tregs following activation of CD3 and CD46 in the presence of IL-2. This illustration not only emphasises the potential significance of C3 dependent adaptive immune function in man, but also provides a link with a body of animal data showing critical dependence of cell-mediated immunity on the complement system, notably through C3.
Update on the role of complement in antibody mediated rejection and accommodation
The complement system plays an essential role in antibody mediated rejection (AMR) via classical pathway activation. The application of C4d staining as a diagnostic marker for AMR exploits this fact [21]. Widespread deposition of C4d associated with AMR is a prognostic marker for reduced long term graft survival [22,23].
Another complement component which has recently gained attention in organ transplantation is CD55, otherwise known as Decay Accelerating Factor (DAF). This molecule is present on the cell surface where it accelerates the decay of C3 and C5 convertases from the classic and alternative pathway of complement to prevent their amplification and self-damaging effect on cells [24].
In a small study of cardiac transplant patients diagnosed with AMR, González-Stawinski et al [25] noted that diffuse endothelial expression of CD55 detected by immunohistochemistry was seen in patients with no sign of allograft dysfunction whereas in patients who had developed allograft dysfunction, they could not detect CD55 expression, suggesting that presence of CD55 has a protective role in AMR. This observation has been reported by another group [26] in cardiac transplant patients, in which subjects without AMR had increasing expression of CD55 and CD59 in their biopsy samples, whereas patients with AMR lost the expression of these molecules in the acute phase, suggesting that their presence could have a protective effect. Pavlov et al [27] demonstrated a similar effect in a murine model of cardiac transplantation, in that daf-/- allografts were rejected faster that WT grafts. They found that the main effect of daf on local complement activation was on the CD8+ T cell response, and accelerated rejection was also seen in recipients who did not have donor specific antibody.
To investigate the association of CD55 expression on renal tubular cells with graft survival in man, Brodsky et al [28] in a review of over 200 renal allograft biopsies performed to assess graft dysfunction, looked for the presence of CD55 and C4d. They examined the relationship of staining with allograft function represented by serum creatinine. They found an inverse relationship, in that patients with no C4d staining had increased expression of CD55, and this was associated with better graft function and longer graft survival. Although unlike the cardiac transplants discussed above, this effect was not significant in the C4d positive group (indicating that CD55 expression in the kidney is not protective of AMR) it represents an important finding suggestive of a protective role of CD55 in a non-AMR setting and that expression of CD55 in peritubular cells could be a marker of renal allograft survival.
The pressures of donor organ shortage and increase in the number of hypersensitized patients due to failing grafts has forced the expansion of transplantation of recipients with HLA or ABO mismatched donor organs. This has been permitted by use of more intense immunosuppressive regimens, including intravenous immunoglobulin (IVIG), removal of alloantibodies and introduction of newer therapeutic agents such as rituximab (a monoclonal antibody against CD20 on B cells) which suppress B cell function [29]. It has been noted, however, that some grafts continue to have good function despite elevated levels of donor specific antibodies. Surprisingly in this group of patients, deposition of C4d is not directly related to graft function or outcome. This phenomenon has been called accommodation, since endothelial cells appear to adapt to the presence of noxious stimuli [30]. Although the concept is widely accepted, its mechanism is only partially understood. Griesemer et al [31] recently investigated the role of complement regulatory protein CD59 in inducing accommodation, in a model using alpha-1,3-galactosyltransferase knock-out (GalT-KO) pigs that produce anti-Gal antibodies (similar to antibody against human blood group antigen). Transplanting four MHC matched kidneys from Gal positive miniature pig to GalT-KO pig using tacrolimus as systemic immunosuppression, the co-authors found that in one of the transplants where the animal had low antibody titres, accommodation had developed, whereas the other 3 kidney transplants were rejected through an antibody mediated mechanism. The expression of CD59 in the accommodated kidney was elevated and deposition of C5b-9 was reduced and the reverse was seen in the rejected kidneys. Although this is a small study, the results if confirmed would suggest a possible mechanism of accommodation and open up new possible targets for therapy.
The inference that accommodation could be related to complement terminal attack complex (C5b-9) activity is also supported by a mouse kidney transplant model [32]. To induce AMR, BALB/c recipient mice were presensitized with skin graft from MHC mismatched C3H donor mice before a kidney transplant was performed. Different therapeutic regimens included a functionally blocking anti-C5 monoclonal antibody in the presence or absence of cyclosporin and/or LF15–0195 (a new analogue, 15-deoxyspergualin (DSG) which is less toxic and more potent than DSG). The new finding is that the group treated with the combination of anti C5 monoclonal antibody, cyclosporin and LF achieved graft survival in excess of 100 days, despite the persistence of anti donor antibodies. Untreated mice lost their grafts in an average of 9 days due to AMR. Other treatment groups which received anti-C5 monotherapy also rejected the kidney in less than 2 weeks. This result favours a role for C5 blockade in inducing accommodation and highlights a potential avenue for clinical translation.
Potential new therapeutic developments
C5 is a potentially interesting therapeutic target. As hinted above, the breakdown product C5a and its receptor on tissue-resident and migratory cells are involved in reperfusion injury and in cell mediated and antibody mediated rejection. In a syngeneic mouse model of kidney transplantation, Lewis et al [33] recently demonstrated that preservation of kidney in University of Wisoncin solution in the presence of C5a receptor antagonist can significantly increase the graft survival. They also looked at the expression of C5a receptor in deceased and living related donor allografts and found the expression to be significantly higher in the former group. They noted that the increased expression of C5aR in cadaveric donors was associated with cold ischaemia time and graft dysfunction. These findings are in line with the gene array analysis from Naesens et al [7], relating complement molecules to the damage inflicted to the organ by cold ischaemia time. An alternative strategy reported by Zheng et al [34] involved the use of C5aR siRNA which was injected into mice 2 days before induction of renal ischaemia. The authors observed a protective effect by silencing of C5aR gene expression.
One of the most promising new therapeutics to prevent rejection is eculizumab. This is an antibody against C5 molecule which inhibits the formation of the membrane attack complex (and C5a) and has been licensed in the UK for treatment of Paroxysmal Nocturnal Haemoglobinuria [35]. In a recent case report, Locke et al [36] described the use of this compound along with IVIG and plasma exchange for a severe case of AMR. Cornell et al [37] also reported interesting results from use of eculizumab in the prevention of AMR. Comparing retrospective biopsy results from a 2 year period with 4 patients given eculizumab has shown that the treatment group have less evidence of endothelial damage despite high levels of DSA and positive C4d staining. Stegall et al [38] reported earlier this year, their experience of cross-match positive recipients treated with eculizumab in comparison with a historical group who received conventional immunosuppression plus plasma exchange based on the DSA titres. After a month, the control group showed 36% incidence of AMR, but the eculizumab group had no sign of rejection with stable graft function despite persistence of DSA titres. These studies suggest that blockade of C5 by preventing the formation of membrane attack complex (MAC) prevents damage to the endothelium and leads to the phenomenon of accommodation. Although these studies have involved only a small number of patients, the results are encouraging and call for larger study populations to clarify this effect.
Investigating early pathway complement inhibition, Ghebremariam et al [39] reported the effect of vaccinia virus complement control protein (VCP) in a rat model of ischaemia-reperfusion injury. VCP is known to inhibit the activation of C3 (thereby preventing the formation of C3b) and hence down regulate the alternative, classical and lectin pathways of complement. They observed reduced injury in the VCP treated animals compared to the PBS control treated group. Although in this study they used systemic VCP for treatment of animals, there could be a case for investigating the effect of treating the donor organ with VCP and assess its protective effect. This way, systemic inhibition of complement activation at the time of transplantation which might be associated with higher risk of infectious complications, is avoided.
Conclusion
A large body of literature is now available that supports the role of complement in various aspects of immune response following organ transplantation. This provides great optimism for future therapeutic targets.
Acknowledgement
The authors acknowledge the support of the MRC Centre for Transplantation, Kidney Research UK and the Wellcome Trust and financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Damman J, Schuurs TA, Ploeg RJ, Seelen MA. Complement and renal transplantation: from donor to recipient. Transplantation. 2008 Apr 15;85(7):923–7. doi: 10.1097/TP.0b013e3181683cf5. [DOI] [PubMed] [Google Scholar]
- 2.Vieyra MB, Heeger PS. Novel aspects of complement in kidney injury. Kidney Int. 2010 Mar;77(6):495–9. doi: 10.1038/ki.2009.491. Epub 2009 Dec 16. [DOI] [PubMed] [Google Scholar]
- 3.Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. 2006 Feb;20(2):217–26. doi: 10.1096/fj.05-4747com. [DOI] [PubMed] [Google Scholar]
- 4.Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997 Apr 15;63(7):968–74. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65:713–718. doi: 10.1111/j.1523-1755.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 6.Shoskes DA, Shahed AR, Kim S. Delayed graft function. Influence on outcome and strategies for prevention. Urol Clin North Am. 2001 Nov;28(4):721–32. [PubMed] [Google Scholar]
- ** 7.Naesens M, Li L, Ying L, et al. Expression of complement components differs between kidney allografts from living and deceased donors. J Am Soc Nephrol. 2009 Aug;20(8):1839–51. doi: 10.1681/ASN.2008111145. Epub 2009 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprehensive unbiased exploration of microarray gene expression comparing live and deceased donor kidneys. It has shown a significant difference in complement pathway mRNA expression between the 2 groups prior to reperfusion and an association between this expression and graft survival.
- 8.Klos A, Tenner AJ, Johswich KO, et al. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009 Sep;46(14):2753–66. doi: 10.1016/j.molimm.2009.04.027. Epub 2009 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Q, Li K, Sacks SH, Zhou W. The role of anaphylatoxins C3a and C5a in regulating innate and adaptive immune responses. Inflamm Allergy Drug Targets. 2009 Jul;8(3):236–46. doi: 10.2174/187152809788681038. Review. [DOI] [PubMed] [Google Scholar]
- 10.Sacks S, Lee Q, Wong W, Zhou W. The role of complement in regulating the alloresponse. Curr Opin Organ Transplant. 2009 Feb;14(1):10–5. doi: 10.1097/MOT.0b013e32831ec551. [DOI] [PubMed] [Google Scholar]
- * 11.Strainic MG, Liu J, Huang D, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008 Mar;28(3):425–35. doi: 10.1016/j.immuni.2008.02.001. Epub 2008 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This interesting paper discusses the mechanism by which anaphylatoxins C3a and C5a influence T cell activation.
- * 12.Peng Q, Li K, Wang N, et al. Dendritic cell function in allostimulation is modulated by C5aR signaling. J Immunol. 2009 Nov 15;183(10):6058–68. doi: 10.4049/jimmunol.0804186. Epub 2009 Oct 28. [DOI] [PubMed] [Google Scholar]; This study describes the role of C5aR activation on DCs and how this affects Tcell stimulation.
- * 13.Weaver DJ, Jr, Reis ES, Pandey MK, et al. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2009 Dec 16; doi: 10.1002/eji.200939333. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; The role of C5a receptor on DC in differentiation of T cells to various subgroups of Th1, Th17 and Tregs are discussed in this study.
- 14.Kemper C, Chan AC, Green JM, et al. Activation of human CD4+cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- * 15.Fuchs A, Atkinson JP, Fremeaux-Bacchi V, Kemper C. CD46-induced human Treg enhance B-cell responses. Eur J Immunol. 2009 Nov;39(11):3097–109. doi: 10.1002/eji.200939392. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigates how CD-46 induced T regs promote antibody production by B ceels through both cell contact and IL-10 secretion.
- * 16.Raedler H, Yang M, Lalli PN, et al. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009 Aug;9(8):1784–95. doi: 10.1111/j.1600-6143.2009.02723.x. Epub 2009 Jun 26. [DOI] [PubMed] [Google Scholar]; Very interesting paper that shows how local production of C5a by endothelial cells results in expansion of CD8+ T cells, potentially affecting T cell cardiac transplant injury.
- 17.Csomor E, Bajtay Z, Sándor N, et al. Complement protein C1q induces maturation of human dendritic cells. Mol Immunol. 2007 Jul;44(13):3389–97. doi: 10.1016/j.molimm.2007.02.014. Epub 2007 Mar 26. [DOI] [PubMed] [Google Scholar]
- * 18.Baruah P, Dumitriu IE, Malik TH, et al. C1q enhances IFN-gamma production by antigen-specific T cells via the CD40 costimulatory pathway on dendritic cells. Blood. 2009 Apr 9;113(15):3485–93. doi: 10.1182/blood-2008-06-164392. Epub 2009 Jan 26. [DOI] [PubMed] [Google Scholar]; This study explains the potential mechanism by which C1q produced by DCs affects both their own maturation and T-cell activation.
- 19.Lin T, Zhou W, Farrar CA, et al. Deficiency of C4 from donor or recipient mouse fails to prevent renal allograft rejection. Am J Pathol. 2006 Apr;168(4):1241–8. doi: 10.2353/ajpath.2006.050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghannam A, Pernollet M, Fauquert JL, et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol. 2008 Oct 1;181(7):5158–66. doi: 10.4049/jimmunol.181.7.5158. [DOI] [PubMed] [Google Scholar]
- 21.Colvin RB. Antibody-Mediated Renal Allograft Rejection: Diagnosis and Pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 22.Kayler LK, Kiss L, Sharma V, et al. Acute renal allograft rejection: diagnostic significance of focal peritubular capillary C4d. Transplantation. 2008 Mar 27;85(6):813–20. doi: 10.1097/TP.0b013e3181669194. [DOI] [PubMed] [Google Scholar]
- 23.Kieran N, Wang X, Perkins J, et al. Combination of peritubular c4d and transplant glomerulopathy predicts late renal allograft failure. J Am Soc Nephrol. 2009 Oct;20(10):2260–8. doi: 10.1681/ASN.2009020199. Epub 2009 Sep 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–78. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Stawinski GV, Tan CD, Smedira NG, et al. Decay-accelerating factor expression may provide immunoprotection against antibody-mediated cardiac allograft rejection. J Heart Lung Transplant. 2008 Apr;27(4):357–61. doi: 10.1016/j.healun.2008.01.008. [DOI] [PubMed] [Google Scholar]
- *26.Tan CD, Sokos GG, Pidwell DJ, et al. Correlation of donor-specific antibodies, complement and its regulators with graft dysfunction in cardiac antibody-mediated rejection. Am J Transplant. 2009 Sep;9(9):2075–84. doi: 10.1111/j.1600-6143.2009.02748.x. Epub 2009 Jul 16. [DOI] [PubMed] [Google Scholar]; Reviewing a large number of cardiac biopsy samples, this paper shows how combined evaluation of C3d and C4d staining is a better index for diagnosis of AMR and also the potential role of CD55 and CD59 in the incidence of AMR.
- 27.Pavlov V, Raedler H, Yuan S, et al. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol. 2008 Oct 1;181(7):4580–9. doi: 10.4049/jimmunol.181.7.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brodsky SV, Nadasdy GM, Pelletier R, et al. Expression of the decay-accelerating factor (CD55) in renal transplants -a possible prediction marker of allograft survival. Transplantation. 2009 Aug 27;88(4):457–64. doi: 10.1097/TP.0b013e3181b0517d. [DOI] [PubMed] [Google Scholar]
- 29.Jordan SC, Peng A, Vo AA. Therapeutic strategies in management of the highly HLA-sensitized and ABO-incompatible transplant recipients. Contrib Nephrol. 2009;162:13–26. doi: 10.1159/000170864. Epub 2008 Oct 31. [DOI] [PubMed] [Google Scholar]
- 30.Böhmig GA, Bartel G, Regele H, Wahrmann M. Prospects and limitations of post-transplantation alloantibody detection in renal transplantation. Hum Immunol. 2009 Aug;70(8):640–4. doi: 10.1016/j.humimm.2009.04.014. Epub 2009 Apr 15. [DOI] [PubMed] [Google Scholar]
- 31.Griesemer AD, Okumi M, Shimizu A, et al. Upregulation of CD59: potential mechanism of accommodation in a large animal model. Transplantation. 2009 May 15;87(9):1308–17. doi: 10.1097/TP.0b013e3181a19afc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rother RP, Arp J, Jiang J, et al. C5 blockade with conventional immunosuppression induces long-term graft survival in presensitized recipients. Am J Transplant. 2008 Jun;8(6):1129–42. doi: 10.1111/j.1600-6143.2008.02222.x. Epub 2008 Apr 29. [DOI] [PubMed] [Google Scholar]
- 33.Lewis AG, Köhl G, Ma Q, et al. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin Exp Immunol. 2008 Jul;153(1):117–26. doi: 10.1111/j.1365-2249.2008.03678.x. Epub 2008 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, Zhang X, Feng B, et al. Gene silencing of complement C5a receptor using siRNA for preventing ischemia/reperfusion injury. Am J Pathol. 2008 Oct;173(4):973–80. doi: 10.2353/ajpath.2008.080103. Epub 2008 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly R, Richards S, Hillmen P, Hill A. The pathophysiology of paroxysmal nocturnal hemoglobinuria and treatment with eculizumab. Ther Clin Risk Manag. 2009;5:911–21. doi: 10.2147/tcrm.s3334. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locke JE, Magro CM, Singer AL, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009 Jan;9(1):231–5. doi: 10.1111/j.1600-6143.2008.02451.x. Epub 2008 Oct 31. [DOI] [PubMed] [Google Scholar]
- 37.Cornell LD, Burns JM, Stegall MD, Mayo Clinic, Rochester MN. Prevention of Endothelial Activation with C5 Inhibition in Positive-Crossmatch Kidney Transplants (+XMKTx). Abstract presented at American transplant Congress 2009. [Google Scholar]
- 38.Stegall MD, Diwan TS, Burns JM, et al. Prevention of Acute Humoral Rejection with C5 Inhibition. Abstract presented at American transplant Congress 2009; Transplantation Surgery, Mayo Clinic, Rochester, MN; Nephrology and Hypertension, Mayo Clinic, Rochester, MN; Laboratory Medicine, Mayo Clinic, Rochester, MN. [Google Scholar]
- 39.Ghebremariam YT, Engelbrecht G, Tyler M, et al. Vaccinia Virus Complement Control Protein (VCP) Improves Kidney Structure and Function Following Ischemia/Reperfusion Injury in Rats. J Surg Res. 2009 Jun 6; doi: 10.1016/j.jss.2009.04.049. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]