Table 2.
In vitro Binding of Amine-linked Analogs at D2R and D3Ra
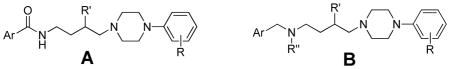 | ||||||||
|---|---|---|---|---|---|---|---|---|
| compound | temp | R | Ar | R′ | R″ | D2R Ki ± S.E.M., nM | D3R Ki ± S.E.M., nM | D2/D3 |
| 13a | B | 2-OCH3 |
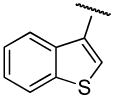
|
H | n-propyl | 69.9±7.3 | 67.9±2.4 | 1 |
| 13b | B | 2,3-diCl |
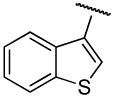
|
H | n-propyl | 162.0±1.2 | 101±27 | 2 |
| 13c | B | 2-OCH3 |
|
H | n-propyl | 196±30 | 71.5±2.0 | 3 |
| 13d | B | 2,3-diCl |
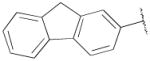
|
H | n-propyl | 269±24 | 129.±30 | 2 |
| 13e | B | 2-OCH3 |
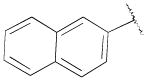
|
H | n-propyl | 119 ± 11 | 80.8±32 | 1.5 |
| 13f | B | 2,3-diCl |
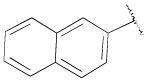
|
H | n-propyl | 313 ± 8.4 | 104±21 | 3 |
| 14a | B | 2,3-diCl |
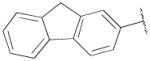
|
OH | H | 2090 ± 370 | 134±23 | 16 |
| 14b | B | 2,3-diCl |
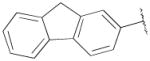
|
F | H | 5270±1900 | 467±60 | 11 |
| 15a | B | 2,3-diCl |
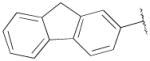
|
OH | Me | 869±140 | 162±32 | 5 |
| 15b | B | 2,3-diCl |
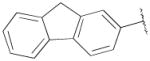
|
F | Me | 2060±320 | 393±38 | 5 |
| 16e | A | 2-OCH3 |
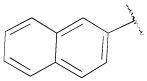
|
H | - | 33.6±5.9 | 0.30±0.06 | 112 |
| 17b,f | A | 2,3-diCl |
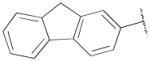
|
H | - | 112 ± 22 | 2.00±0.4 | 56 |
| 18c,g | A | 2-OCH3 |
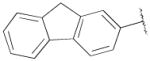
|
H | - | 55.4±5.3 | 0.30±0.1 | 185 |
| 19b,h | A | 2,3-diCl |
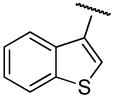
|
H | - | 64.7±8.9 | 0.80±0.2 | 81 |
| 20b,i | A | 2,3-diCl |
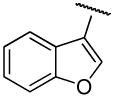
|
H | - | 44.8±11 | 0.80±0.3 | 56 |
Binding inhibition values determined using HEK 293 cells transfected with hD2LR or hD3R dopamine receptors and125I-IABN radioligand as described.5,6
data previously reported in ref. 5;
data previously reported in ref. 6.
BP 897 (N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-2-naphthamide);
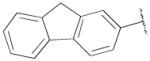
NGB 2904 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide);
PG 576 (N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide);
PG01032 (Benzo[b]thiophene-2-carboxylic acid {4-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-butyl}-amide);
PG01059 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)benzofuran-2-carboxamide).
