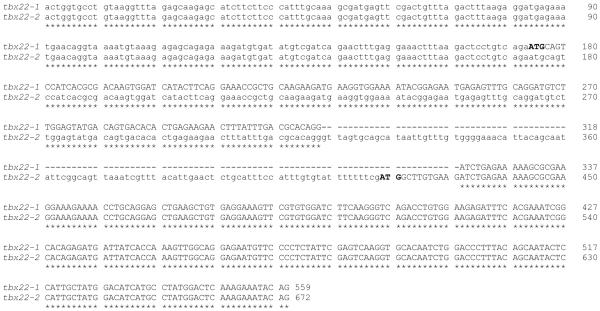
Figure 2. Detailed genomic organization of zebrafish tbx22-1 and tbx22-2.
A) Nucleotide sequence comparison of the first three exons for tbx22-1 and the first two exons for tbx22-2 cDNA sequences distinguish the alternatively spliced isoforms. A Clustal W 1.83 alignment of the zebrafish tbx22-1 and tbx22-2 nucleotide sequences is shown. Coding sequence is shown in upper case, and noncoding is shown in lower case font. Asterisks indicate nucleotide identity, and dashes indicate gaps in the alignment.
B) Multisequence alignments of the predicted amino acid sequences for zebrafish tbx22-1 and tbx22-2, as compared to human and mouse Tbx22 proteins, are shown in nexus format. The T-box domain (as defined in Muller and Herrmann, 1997) is indicated in bold type. Functionally conserved amino acids within the T-box domain are indicated as follows: amino acids involved in Tbx22 dimerization are underlined, and amino acids involved in DNA binding are italicized. Dots represent identity, and dashes represent gaps in the alignment. Human TBX22, ENSP00000362393; Mouse Tbx22, ENSMUSP00000033593.