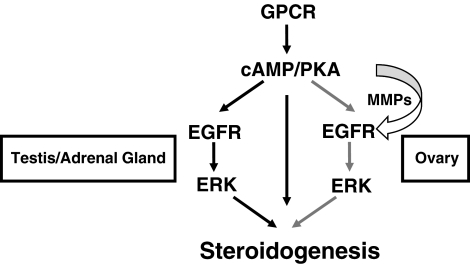
Fig. 8.
Model for steroidogenesis in the ovary, testes, and adrenal gland. In all three steroidogenic tissues, LH or ACTH binds its respective GPCR to activate Gαs and increase cAMP levels. Elevated cAMP then leads to PKA activation. In Leydig or adrenal cells, PKA then regulates two processes, both of which are required for normal steroidogenesis (black arrows). The first (middle arrow) likely involves activation of CREB and up-regulation of StAR and steroidogenic enzymes as well as direct activation of StAR through yet unknown mechanisms. The second (left black arrows) involves trans-activation of the EGFR, Erk activation, and subsequent StAR phosphorylation/activation. Although MMP are activated and EGFR ligands released, EGFR trans-activation occurs in an MMP- and ligand-independent, or intracellular, mechanism in Leydig and adrenal cells. In contrast, steroidogenesis requires cross talk between mural granulosa cells (which express LHR) and cumulus granulosa cells (which make progesterone and other steroids) in the ovary. Here, FSH activates PKA in cumulus granulosa cells to trigger the first signal (equivalent to the middle arrow). LH then triggers MMP-mediated release of EGFR ligands from mural granulosa cells, leading to ligand-dependent trans-activation of EGFR (right gray arrows), Erk activation, and StAR phosphorylation/activation in cumulus granulosa cells. Once activated, StAR promotes steroidogenesis in all three tissues.