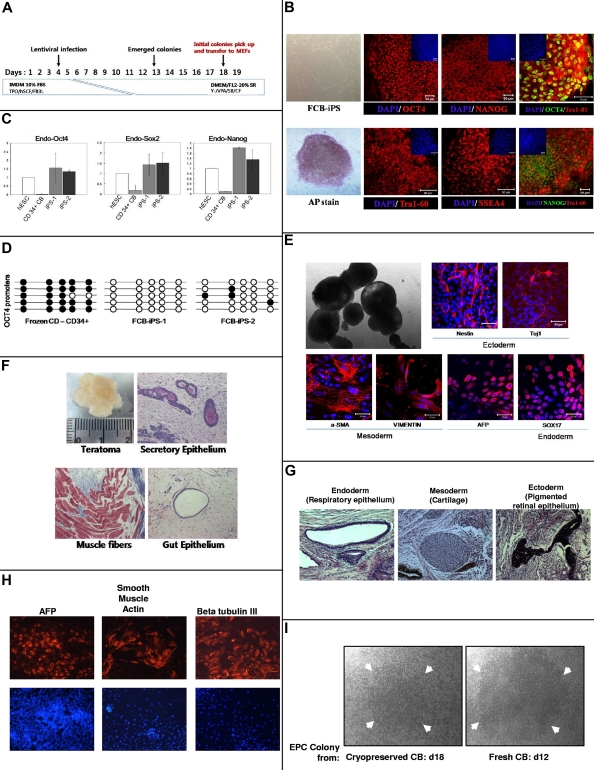
Figure 2.
Reprogramming of 21-year-old frozen human CB CD34+ cells to iPS cells (A-H) and recovery of ECFCs (I). The phase contrast images in panels B (FCB-iPS and AP stain), E (top left image), and I were viewed using a Zeiss Axiovert 25 CFL inverted microscope with a 5× CP-ACHROMAT/0.12 NA objective. Images were acquired using a SPOT RT color camera (Diagnostic Instruments) with the manufacturer's software. Phase contrast images were taken with air objectives. The confocal images in panels B and E were viewed with a Zeiss Axiovert 100 LSM 510 confocal microscope using as objective a C-Apochromat (at 40×/1.2W corr) or Plan-Apochromat (at 10×/0.45). Images were taken by epifluorescence detector and transillumination detector and processed using Zeiss LSM Image Browser Version 3.5.0.376 software. All the images were taken at room temperature. Cells were mounted in proLong Gold antifade mounting reagent with DAPI (Invitrogen) and confocal images were taken using water immersion objectives. (A) Schematic representation of strategy used at IU to reprogram CD34+ cells from frozen human CB. TPO indicates Thrombopoietin; hSCF, stem cell factor; Flt2L, fms-like tyrosine kinase 3 ligand; Y, Y-27632; VPA, valporic acid; and SB, SB202190. (B) Immunocytochemistry for pluripotency markers OCT4 (Alexa 448 or 546 nm), NANOG (Alexa 488 or 564 nm), TRA-1–60 (Alexa 564 nm), SSEA4 (Alexa564 nm) and alkaline phosphatase (AP). Small panels on top part of subpanels show total cell content per field stained with DAPI. Scale bars represent 50 μm. (C) Quantitative RT-PCR analysis for expression of endogenous ES cell-marker genes OCT4, SOX2, and NANOG in hESC line H9, 2 iPS cell lines generated, and parental CD34+ cells. Specific primers were designed to probe for 3′ untranslated region (Endo) to measure expression of the endogenous gene. Individual PCR reactions were normalized against β-actin and plotted relative to expression levels in hESCs. (D) Each horizontal row of circles represents an individual sequencing reaction for a given amplicon. Open and filled circles, respectively, represent unmethylated and methylated CpGs dinucleotides. (E) Morphology of 10-day embryoid body (EB) under phase contrast microscopy (top left). Immunostaining of frozen CB (FCB)–iPS derivatives on day 10 of differentiation revealed expression of ectodermal (Nestin and Tuj1), mesodermal (α-SMA and Vimentin), and endodermal (AFP and Sox17) marker proteins (all with Alexa 546nm). Nuclei are stained with DAPI (blue). Scale bars represent 50 μm. (F) Teratomas derived from immunodeficient mice injected with FCB-iPS cells shows tissues representing all 3 embryonic germ layers, including secretory epithelium (ectoderm), muscle fibers (mesoderm), and gut epithelium (endoderm). Samples from the teratomas were paraffin-embedded and serially sectioned (5 μm) using a microtome (Leica Microsystems). To analyze the 3 germ-layer lineage cells derived from injected FCB-iPS cells in the teratomas, sectioned slides were histologically examined by hematoxylin and eosin for the gut epithelium and special stains as follows: PAS stain for the secretory epithelium, and Masson trichrome stain for muscle fibers. Images were taken with a Nikon Eclipse 50i equipped with a digital camera (Infinty 2 Megapixel; Lumenera Corp) at a magnification of Plan Fluor 10×/0.30 and analyzed using the i-solution image analysis program (iMT; i-Solution Inc). Teratoma images were taken with air objectives. Results in panels B through F were from studies performed at IU. Results in panels G and H were from another 21-year cryopreserved CB sample sent to, thawed, and separately reprogrammed at Johns Hopkins Medical Center12 after isolation of CD34+ cells. For studies in panels G and H, a Nikon Eclipse TE2000-U microscope was used, with ELWD Plan Fluor, NA:0.45 at 25°C. For panel G, fixed slides were used with H&E staining, and for panel H, PBS plus 0.1% BSA was used with Alexa 555 and DAPI. Images were taken with Imaging Micropublisher 5.0 (Q Imaging) camera with Q Capture Version 3.1.2 (Q Imaging) software. Markers for teratoma formation in vivo (G) and for differentiation of EBs from reprogrammed iPS cells in vitro (H). (I) Representative ECFC colony from MNCs isolated from 21-year CB defrosts and cultured for 18 days (left) compared with colony from freshly obtained CB cultured for 12 days. Arrows denote size of colonies. Note that even after 18 days culture of frozen CB-derived colonies, the colony size is not as large as that of colony from fresh CB at 12 days of culture.