Fig. 7.
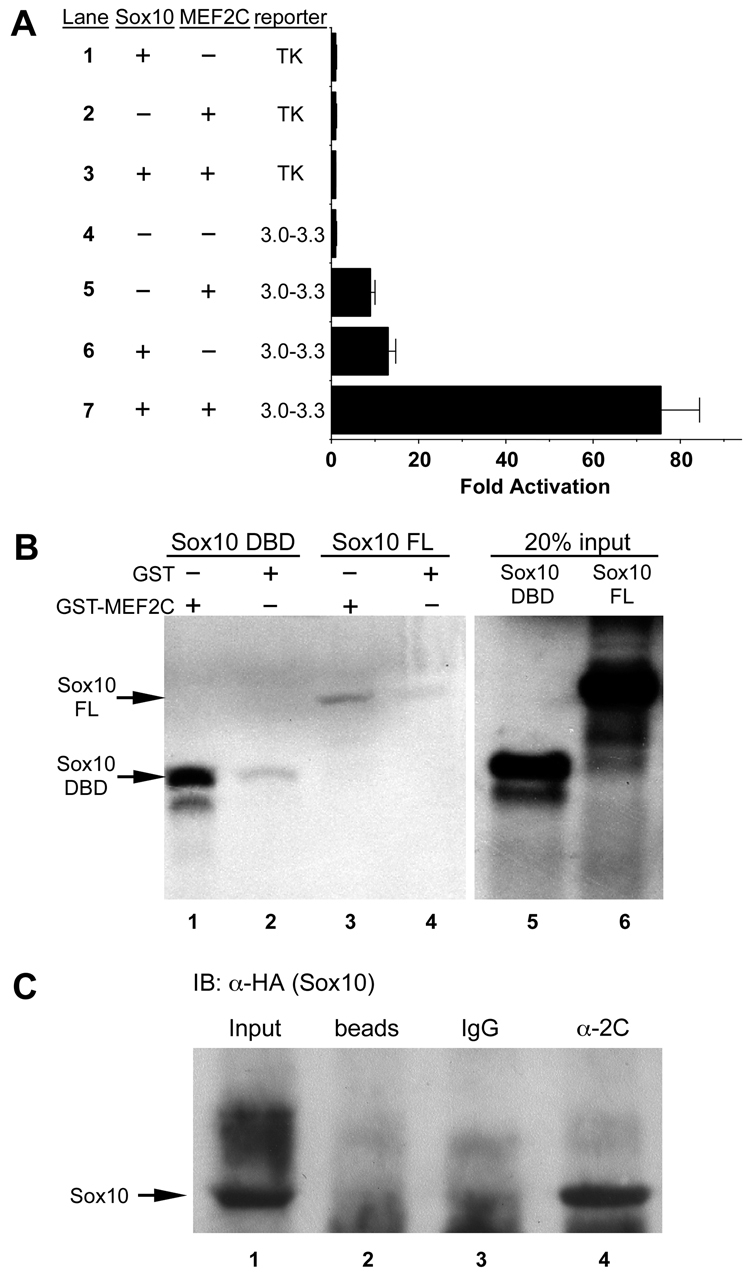
MEF2C and SOX10 physically interact and synergistically activate the Mef2c-F1 enhancer. (A) Co-transfection of expression plasmids for MEF2C-VP16 and SOX10 with the Mef2c[3-3.3]-F1-TK-lacZ reporter plasmid resulted in synergistic activation of the Mef2c-F1 enhancer (lane 7) compared with transfection of the reporter with MEF2C-VP16 (lane 5) or SOX10 (lane 6) alone. No activation of the reporter with empty expression vectors or of the parental TK-lacZ reporter was observed (lanes 1-4). Data are expressed as the mean fold activation + s.e.m. from three independent transfections and analyses. (B) Agarose conjugated to either GST-MEF2C (lanes 1, 3) or GST alone (lanes 2, 4) were used as bait in pull-down assays with radiolabeled DNA binding domain (DBD) of SOX10 (lanes 1, 2) or with radiolabeled full length (FL) SOX10 (lanes 3, 4). Twenty percent of the input amount of radiolabeled SOX10 DBD and SOX10 FL are included in lanes 5 and 6, respectively. (C) MEF2C and SOX10 interact in vivo in B16F10 melanoma cells. MEF2C- and SOX10[HA]-transfected cells were subjected to immunoprecipitation with anti-MEF2C followed by western blot analysis with anti-HA (SOX10). SOX10 was only detected following precipitation with anti-MEF2C (α-2C, lane 4) but not with isotype-matched IgG (lane 3) or with beads alone (lane 2). Lane 1 contains input sample only for western blot (no prior immunoprecipitation) as a positive control for SOX10[HA] detection.
