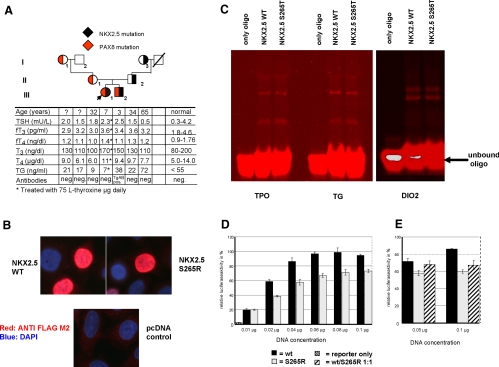
Fig. 1.
A, Pedigree of the family. The index patient is indicated by an arrow. The transmission of the NKX2.5 mutation is shown by a filled black area within each symbol, whereas the segregation of the PAX8 promoter mutation by a corresponding red area. Results of thyroid function tests are aligned with each symbol. B, Nuclear localization of S265R. HeLa cells were transfected with plasmids expressing the WT NKX2.5 and the mutant NKX2.5 (S265R). The NKX2.5 proteins were visualized by immunofluorescence microscopy as described in Materials and Methods. Both proteins (WT and S265R) are localized in the nucleus. C, EMSA with various NKX2.5 promoter binding sites. Oligonucleotides containing the NKX2.5 binding sequences of the TPO, TG, and DIO2 gene promoters were synthesized and labeled with an infrared dye (IRD700). The in vitro synthesized NKX2.5 proteins were incubated with the IRD700 labeled oligonucleotides and separated on a 4% native polyacrylamide gel and visualized with the Odyssey (Li-Cor, Lincoln, NE). D and E, Functional activity of the mutant NKX2.5 tested using the TG-luciferase reporter gene in HeLa cells. HeLa cells were transiently transfected with 0.25 μg TG-luciferase and various amounts of WT or mutant NKX2.5 expression constructs. At all DNA concentrations tested, the NKX2.5 mutant (gray bars) shows an approximately 30% reduced luciferase activity when compared with the WT (black bars). The empty vector control is marked as a hatched bar (D). When cotransfected in equal amounts (hatched bars), S265R exerts a dominant-negative effect on the WT (E).