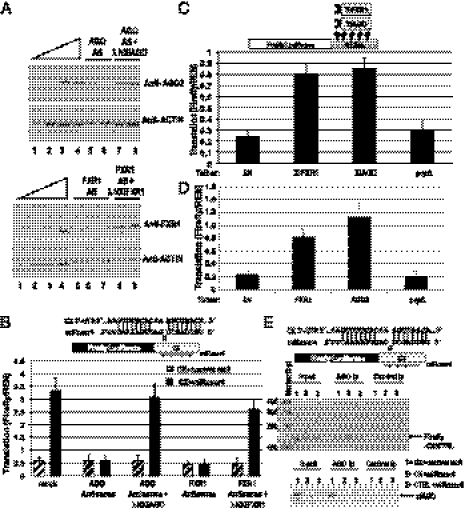
Fig. 3.
FXR1 and xlAGO are required for microRNA-mediated up-regulated translation in oocytes. (A) Sixteen hours of DNA antisense treatment decreases endogenous FXR1 and AGO protein levels in the oocyte, which were rescued by expression of in vitro-transcribed mRNAs encoding λN-tagged Xenopus FXR1 and AGO, respectively, as observed by Western blotting (SI Methods and Fig. S3D). Actin served as a loading control. Twofold dilutions (12.5–100%) of mock-treated immature oocyte extract (lanes 1–4) are shown: Upper (AGO) and Lower (FXR1). Lanes 5–6 and 7–8 (Upper) are duplicate samples, as are lanes 5–7 and 8–9 (Lower). (B) MiRcxcr4 activates translation of the CX reporter mRNA expressed from a plasmid when coinjected into the nuclei of immature, folliculated oocytes (mock). Antisense reduction of Xenopus AGO or FXR1 levels abrogates activation of the CX reporter, which can be rescued by expression of the λN-tagged resistant clones. (C) Expression of λN-tagged Xenopus FXR1 or AGO (XlFXR1 and XlAGO) mRNAs into immature oocyte nuclei causes translation activation of the coinjected tethered reporter. λN and the λNprpΔ mutant form of human AGO2 are controls. (D) Plasmids expressing λN-tagged human FXR1 or AGO2 coinjected into immature oocyte nuclei cause translation activation of the tethered reporter. In vitro-transcribed, capped XlFXR1 and XlAGO mRNAs (A–C), and plasmids expressing hAGO2 and hFXR1 (D) were used for protein expression; experiments in C and D used in vitro-transcribed capped tethered reporter. In B–D, Firefly luciferase values were normalized against REN. (E) RT-PCR analysis (Upper) of RNAs extracted from anti-AGO2 or control anti-FLAG coimmunoprecipitates (Ip) from oocyte nuclear extracts coinjected with the CX or CTRL reporters and the specific miRcxcr4 or the control microRNA, let-7a, reveals significant association of CX but not CTRL mRNA with xlAGO in the presence but not absence of miRcxcr4. (Lower) Western blot of AGO protein in input and immunoprecipitation (Ip) lanes.