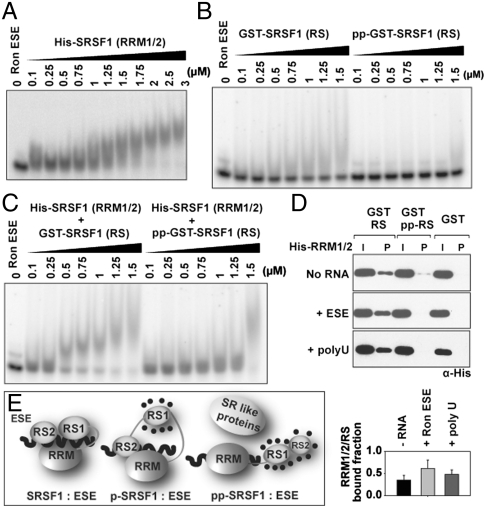
Fig. 2.
Unphosphorylated RS domain interacts with ESE∶SRSF1 (RRM1/2). (A) EMSA showing the binding of SRSF1 (RRM1/2) to Ron ESE. (B) EMSA analysis of ESE mixed with GST-RS (197–248) and GST-pp-RS (197–248). (C) EMSA showing the binding of SRSF1 (RRM1/2) to GST-RS and GST-pp-RS. (D) GST pull-down assay showing binding between His-SRSF1 (RRM1/2) (2 μg) and GST-RS/GST-pp-RS (2 μg) in the absence or presence of Ron ESE or poly U13. Input and bound proteins were detected by the Western blotting using anti-His Ab. Bound fractions was quantitated from three independent experiments (bottom). (E) The model depicting ESE binding to SRSF1 in its different phosphorylated states.