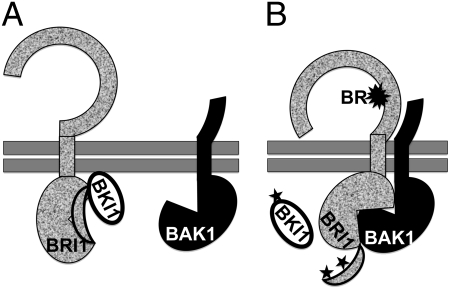
Fig. 1.
A “double-lock” model for stabilizing a BR-triggered BRI1–BAK1 complex. Details are provided in the text. Double horizontal bars indicate the plasma membrane, the crescents mark the autoinhibitory C-terminal end, and the stars mark phosphorylation. (A) In the absence of BR, neither BRI1 nor BAK1 is active. The autoinhibitory C terminus of BRI1 and BKI1 binding to BRI1 prevents the BRI1–BAK1 interaction. (B) BR binding to the ligand-binding domain of BRI1 not only triggers a conformational change in its ECD to allow its low-affinity dimerization with the BAK1 ECD but also causes a structural rearrangement in the BRI1 KD to activate its kinase activity. The slightly activated BRI1 autophosphorylates to lease its autoinhibitory C terminus and transphosphorylates to dissociate BKI1 from the plasma membrane, thus enabling physical docking of the KDs of BRI1 and BAK1 to form a stable receptor–coreceptor complex.