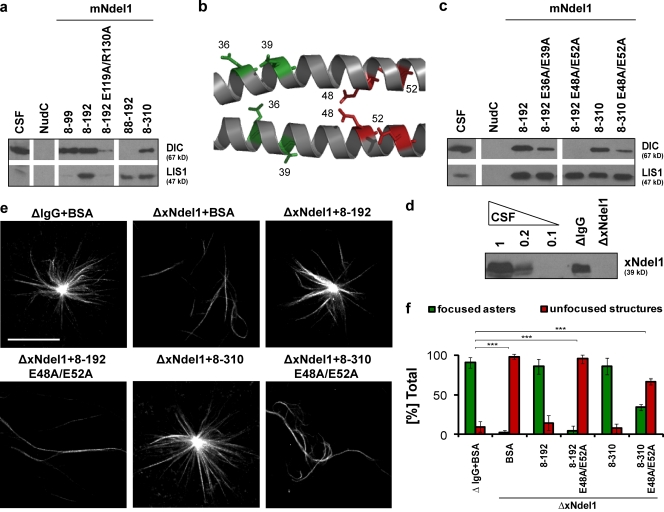
Figure 3.
Dynein interacts with polar residues within the coiled-coil domain of Ndel1. (a) Immunoblot analysis of dynein IC (DIC) and LIS1 pulled down from CSF-arrested Xenopus egg extract by the indicated recombinant mouse Ndel1 proteins. (b) Multiple conserved glutamic acids extend form the coiled-coil backbone of Ndel1; residues shown in color were mutated, residues shown in red are the most critical for dynein binding; image created in Pymol (http://www.pymol.org/) based on the structure of mNdel18–192 (Protein Data Bank accession no. 2v71). (c) Immunoblot analysis of dynein IC (DIC) and LIS1 pulled down from mitotic Xenopus egg extract by mutated mNdel18–192 and mNdel18–310 proteins. (d) Level of Ndel1 depletion from CSF-arrested egg extract with affinity-purified polyclonal α-Ndel1 antibodies analyzed by Western blot. (e) mNdel18–192 E48A/E52A (8–192 E48A/E52A) and mNdel18–310 E48A/E52A (8–310 E48A/E52A) proteins do not significantly rescue aster formation. Representative images of predominant phenotypes from each condition are shown. (f) Quantification of over 100 microtubule structures from three independent experiments after Ndel1 depletion and rescue with mouse Ndel1 variants; asterisks indicate statistically significant differences (Student’s t test with P < 0.05; *** indicates P < 0.0005). Bar, 20 µm.