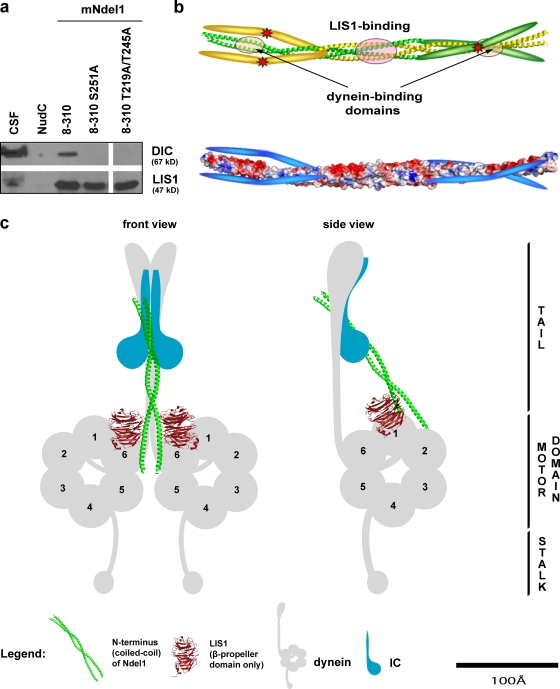
Figure 6.
Proposed role for the regulation of Ndel1 ability to act as a scaffold to promote the interaction between dynein and L IS1. (a) Ndel1 mutants in Aurora A consensus site (S251A) and Cdk1/Cdk5 sites (T219 and T245) abolish the interaction with dynein in a pull-down assay. (b, top) A ribbon model of a nascent Ndel1 tetramer where one dimeric coiled-coil is green and the second is yellow. Note that the structure of the C terminus has not been solved and is presented in cartoon format. The asterisks indicate potential phosphorylation sites. (b, bottom) Space-filling model colored to show charge distribution of Ndel1 in the presumptive autoinhibited form. The C terminus has a pI of 9.8 and is therefore depicted schematically as blue. We propose that in the unphosphorylated state the two Ndel1 dimers interact through the LIS1-binding region, as seen in the crystal structure. In this conformation the highly basic C-terminal tail inhibits the interaction between Ndel1 and dynein by binding the highly acidic (pI 4.5) dynein interaction region on the coiled-coil as shown. (c) Proposed model of the tripartite interaction between dynein, LIS1, and the N terminus of Ndel1. The proteins are all drawn approximately to scale, although their precise locations are not known.