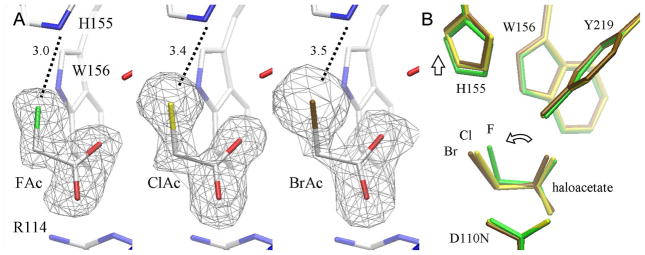
Figure 5. RPA1163 Asp110Asn mutant in complex with fluoro-, chloro- and bromoacetate.
(A) The binding of FAc, BrAc and ClAc in the active site of the Asp110Asn mutant. FAc is bound at full occupancy, but only ~75% saturation for ClAc and BrAc are observed. The residual electron densities on O2 of ClAc and BrAc are both modelled as a chloride ion at ~25% occupancy (Supporting Information, Figure S3). The omit Fo−Fc electron density maps are contoured at 4.5 (FAc), 3.0 (ClAc) and 2.8 (BrAc) σ. (B) The superposition of the FAc, ClAc and BrAc complexes (in green, yellow and brown, respectively) using all Cα atoms of the protein subunits. Only His155 is displaced, albeit minimally, by the increasingly larger halide of the substrate (vertical arrow). The binding of the larger halogen atom forces a slight tilt to the entire haloacetate substrate thereby keeping the halogen atom further out of the active site (curved arrow).