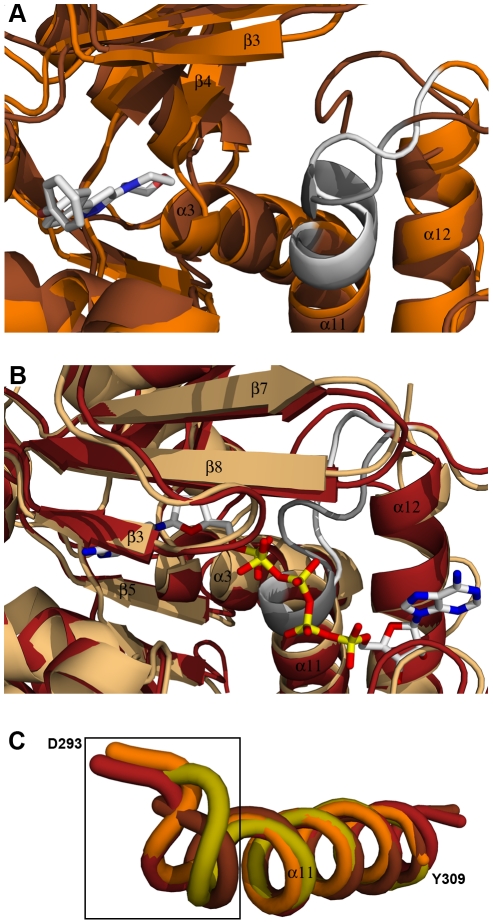
Figure 4. Anion hole formation in TbrAK.
Panel A: Superposition of apo TgoAK (1LIO, open conformation, brown) and the complex of TbrAK and compound 1 (2XTB, open conformation, orange) showing the area involved in the formation of the anion hole. The P-loop heptad (D293-D299) forming the anion hole is shown in white for TbrAK-compound 1 and in gray for TgoAK. Although exhibiting an open conformation, the P-loop heptad in the TbrAK-compound 1 complex is not integrated anymore in helix α11. Panel B: Superposition of TgoAK in complex with adenosine and AMP-PCP (1LII, closed conformation, rust-red) and TbrAK-AP5A (3OTX, closed conformation, light orange) focusing on the area of the anion hole. The P-loop heptad is shown in white for TbrAK-AP5A and in gray for TgoAK. Both P-loop heptads are integrated in helix α11, however, the heptad in TbrAK-AP5A is distorted, most likely due to the presence of the additional phophates in AP5A. Panel C: Superposition and close-up view on helix α11 and the corresponding P-loop heptad of selected adenosine kinases. For the sake of clarity only the first amino acid of the TbrAK heptad (D293) and the last amino acid of helix α11 in TbrAK (Y309) are labeled. The selected structures are TbrAK-compound 1 (2XTB, open conformation, orange), TgoAK in complex with adenosine and AMP-PCP (1LII, closed conformation, rust-red), the apo TgoAK (1LIO, open conformation, brown), and the structure of HsaAK in complex with the alkynylpyrimidine inhibitor (2I6B, open conformation, olive). The black square indicates the area of anion formation at the N-terminus of helix α11. With the exception of TbrAK-compound 1, the structures exhibiting an open conformation have their P-loop heptads integrated in helix α11, while upon substrate binding the first turn of helix α11 unfolds to form the anion hole.