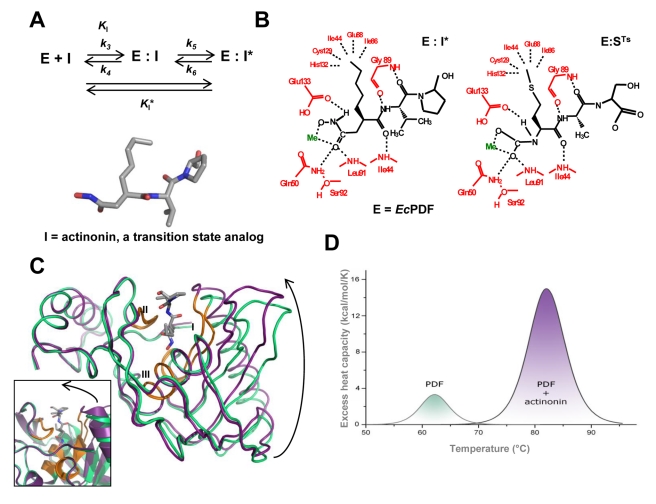
Figure 1. Slow, tight-binding inhibition of PDF by actinonin induces conformational change in the protein.
(A) Inhibition by a two-step mechanism, involving a tightening of the initial enzyme-inhibitor complex (E·I) to form a more stable complex (E·I*), with the chemical structure of actinonin (I), the natural inhibitor of PDF enzymes (E). (B) Structures of EcPDF bound to actinonin (left) and to the transition state resulting from the cleavage of its substrate, Fo-Met-Ala-Ser (right) [34],[35]. (C) Superimposition of free and actinonin-bound AtPDF indicated in green and purple, respectively. The three conserved motifs of the PDF enzymes family are indicated in orange and numbered I, II, and III. Molecules A of both models were superimposed, resulting in an r.m.s.d. of 0.9 Å for 100% of the Cα. Left inset, close-up comparison of the open and closed forms figured in the ribbon representation. (D) Baseline-corrected DSC thermograms of free and actinonin-bound WT AtPDF recorded under the same experimental conditions.