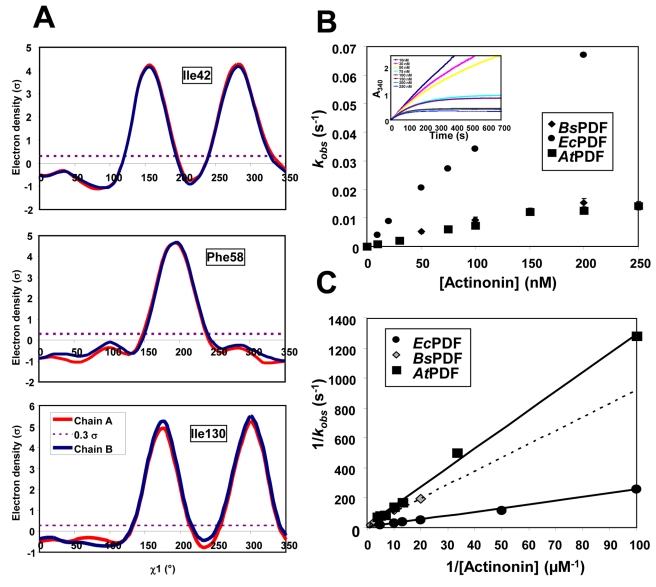
Figure 4. Evidence for an induced fit in crystalline and solution states of AtPDF.
(A) Absence of evidence for alternative conformers in the crystalline state of AtPDF. Ringer plots of electron density (r) versus χ1 angle for representative residues of the 3D apo-structure of AtPDF. Data were obtained with the 3M6O, 3PN2, and 3PN3 datasets (2.0 and 1.3 Å resolution, respectively, see Table S1). The secondary peaks in the Ile residues are observed because Ile is a branched amino acid. To reveal an alternative conformation with Ile, three peaks should be observed. (B) kob s is a saturable function of actinonin with various PDFs, including AtPDF. Data obtained for kob s, the experimentally observed pseudo-first-order rate constant for the approach to equilibrium between the free components and the binary PDF-actinonin complex, were obtained at various concentrations of actinonin in the presence of EcPDF, AtPDF, and BsPDF2. A direct plot is shown. Inset, time-course measurement of deformylation as a function of varying actinonin concentrations. (C) Inverted plot of the data in panel B, which is expected to be a straight line if the kobs is >>k6 in the case of induced fit [19]. The correlation coefficient of each line is 1.00, 0.99, and 1.00 for AtPDF, BsPDF2, and EcPDF, respectively, indicative of the accuracy of the conclusion.