Abstract
Ionizing radiation causes many types of DNA damage, including base damage and single- and double-strand breaks. Photons, including X-rays and γ-rays, are the most widely used type of ionizing radiation in radiobiology experiments, and in radiation cancer therapy. Charged particles, including protons and carbon ions, are seeing increased use as an alternative therapeutic modality. Although the facilities needed to produce high energy charged particle beams are more costly than photon facilities, particle therapy has shown improved cancer survival rates, reflecting more highly focused dose distributions and more severe DNA damage to tumor cells. Despite early successes of charged particle radiotherapy, there is room for further improvement, and much remains to be learned about normal and cancer cell responses to charged particle radiation.
Keywords: DNA damage, ionizing radiation, cancer therapy, gene expression, mutagenesis
1. Introduction
Radiation biology has a long history beginning shortly after the discovery of X-rays by Roentgen in 1895 with reports of X-rays affecting skin in 1896, and inducing skin cancer in 1902. The genetic effects of ionizing radiation (IR) were first explored in Muller’s X-ray experiments with Drosophila [1]. X-rays are generated by accelerating electrons onto a target such as the original cathode ray tube used by Roentgen. X-rays are a class of photons with sufficient energy to create ionization in materials through which they are passing. γ-rays are generally higher energy photons that are emitted through the radioactive decay of elements such as 60Co and 137Cs. X-rays and γ-rays create initial ionizing events that liberate electrons which continue to produce secondary ionizations until they stop. In general the pattern and distribution of these ionizations is widely dispersed within the irradiated material.
In 1919, Ernest Rutherford first demonstrated the existence of the proton [2], opening up studies of many different forms of IR based on charged particles. Such particles range from protons (hydrogen atoms stripped of their single electron) and α particles (He nuclei) to heavy, high energy particles (HZE) such as carbon (12C) and iron (56Fe) ions. These belong to a family of particles called hadrons that refers to their ability to participate in nuclear interactions in addition to atomic interactions based on charge. For decades, the nature, behavior and practical applications of hadrons has captivated physicists and scientists from many other disciplines.
HZE particles create ionization immediately and continuously as they penetrate matter. Because of their large mass, they travel in straight trajectories with a relatively well defined stopping point or range. The pattern of HZE energy deposition is characterized by a dense core of ionization that is localized along the trajectory of the particle [3]. Linear energy transfer (LET) reflects the rate at which ionization is produced along the track of charged particles and has dimensions of energy per unit length (e.g., keV/μm). Electrons have sparse ionizations along the track (~ 0.2 keV/μm) and are classified as low LET radiation. This classification also applies to photons that produce sparsely ionizing electrons, whereas HZE particles can have a LET >100 keV/μm and are classified as high LET radiations.
The biological response to IR is measured with respect to absorbed dose which is operationally defined as the energy absorbed in a volume of material divided by the mass of the volume. Dose is expressed in units of Gray (Gy) which is equivalent to 1 Joule/kg. It was soon recognized that some types of radiation were more effective at killing cells than others. The concept of relative biological effectiveness (RBE) was created to quantify this phenomenon. RBE is the ratio of the dose of a reference radiation (photons) to the dose of the test radiation to produce the same biological endpoint. To a first approximation, RBE increases with increasing LET.
X-ray beams can be produced by compact machines where electrons with energies from 5–200 keV are incident upon a target in an enclosed vacuum tube. The emerging photons have a distribution of energies depending on the energy of the incident electrons and beam energies are indicated as 5 kV, 50 kV, 200 kV etc. Diagnostic imaging with X-rays is usually performed in the range 30–150 kV. γ-rays are emitted with fixed energies that can range from 50 keV to 3 MeV depending on the radioactive isotope. Modern clinical linear accelerators produce external high energy electron beams ranging from 4 MeV to 25 MeV that can be steered to an internal target to produce beams that are referred to as MV photons depending on the energy of the accelerated electrons (e.g., 4 MV, 25 MV).
Hadron beams have been produced at particle accelerator research facilities for many decades. In the US, Fermilab accelerates protons to 2,000,000 MeV (2 TeV) and the Large Hadron Collider in Europe is colliding counter rotating proton beams at 8 TeV. Hadron beams are also being developed for basic biological research and clinical applications for cancer therapy. The two therapeutic modalities for hadron beams are protons from 70–250 MeV and carbon ions from 200–430 MeV. Because relatively simple systems are used to generate X-rays and γ-rays, the vast majority of biological studies of IR over the past century have focused on photons. In this mini-review we focus on the radiobiological and therapeutic aspects of charged particle hadron radiation and highlight different physical and biological effects of photon and hadron radiation.
2. Energy Deposition Patterns of Photon and Hadron Radiation
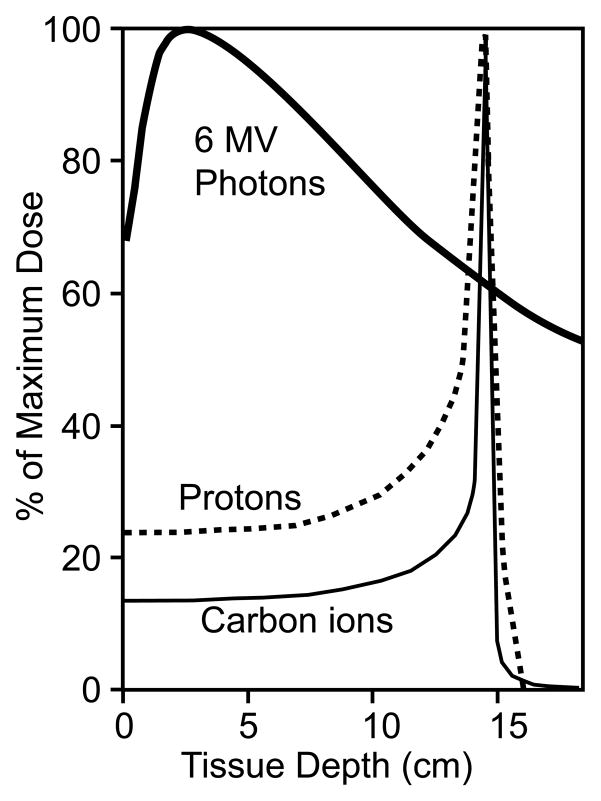
As mentioned above, X-ray and γ-ray photons deposit energy in tissue in a highly dispersed manner, characterized as low “linear energy transfer” (LET). LET is the amount of energy (expressed in electron volts) deposited (or lost) per micrometer (keV/μm), and IR can be either low LET (sparsely ionizing) or high LET (densely ionizing). Photons are low LET radiation, displaying a very broad energy distribution in tissue, and the peak dose is located relatively close to the surface [3] (Fig. 1). Hadron particles may be either low or high LET, but in either case, hadrons interact with matter and deposit energy quite differently than photons [3] (Fig. 1). As hadron particles slow from collisions with matter they release small amounts of energy along the track until they reach a critical speed at which point they decelerate rapidly, depositing a large fraction of their energy in a very narrow zone termed the Bragg peak, first described in 1907 [4]. Beyond the Bragg peak, the energy release diminishes very rapidly, thus tissue beyond the Bragg peak receives little or no radiation dose. The broad distribution of photon energy in tissue, and the peak deposition near the surface, makes it difficult to effectively treat deep-seated tumors and spare normal tissue. To overcome these limitations, photon beams are typically directed toward tumors from several directions and beam intensity is modulated (intensity modulated radiation therapy or IMRT), “conforming” a high dose to the tumor and minimizing the dose surrounding normal tissue.
Fig. 1.
Depth dose distributions for 6MV photons, stopping protons and carbon ions. The plots are normalized to 100% at the depth corresponding to maximum dose. Adapted from ref. [3].
The physical properties of hadron radiation provide several significant benefits in radiotherapy. First, large amounts of energy can be accurately deposited into tumors at various depths. Second, there is less damage to surrounding normal tissue, particularly beyond the distal boundary of the tumor due to the rapid decrease in energy deposition distal to the Bragg peak [5,6]. In addition, proximal tissue, while receiving a significant dose, is exposed primarily to less harmful low LET radiation (see below). These properties allow clinicians to spare sensitive tissues, such as the spinal cord, when treating nearby tumors. Carbon ion beams also have smaller penumbras (less lateral spread) than photons or protons, increasing the accuracy of beam delivery to tumor targets [7]. Although protons and carbon ions both show sharp Bragg peaks, a key difference is that protons are low LET radiation, like photons, whereas carbon ions are high LET radiation which creates more complex damage in DNA and other cellular structures and molecules, yielding greater tumor killing, as discussed in the next section.
3. LET, RBE, and the Oxygen Enhancement Ratio
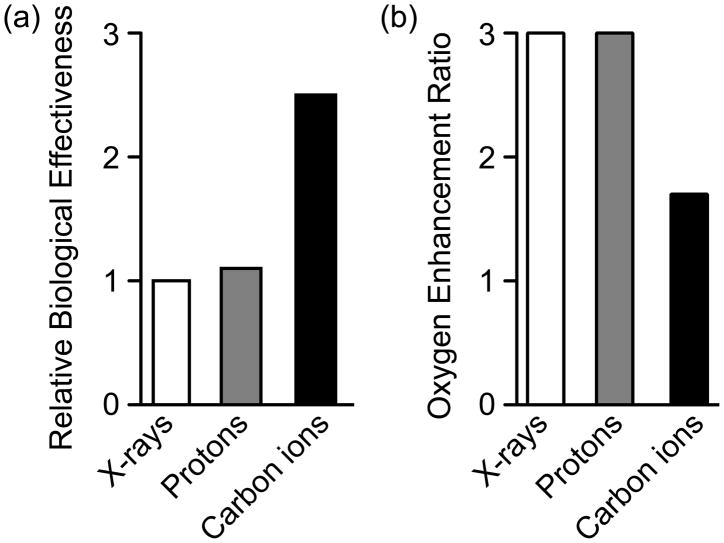
RBE compares the efficiency of different types of radiation relative to γ-rays in producing a defined biological effect (typically cell killing) [8–10]. The average RBE for protons is ~1.1 [11] while the average RBE for carbon ions is much higher, estimated to be 2.5 to 3 (Fig. 2a), although values as high as 5 have been reported [12]. RBE can vary considerably within the volume of a tumor due to factors such as beam spread, depth of the tissue and numerous other parameters [5]. The greater RBE with carbon ions is largely a reflection of its high LET, which produces more complex DNA damage that is refractory to repair [13,14], and can stimulate apoptosis [15,16]. Cellular responses to high LET radiation are the focus of much biological research, due to the clinical relevance of DNA damage in cancer therapeutic strategies.
Fig 2.
RBE (a) and OER (b) of X-rays, protons, and carbon ions. Adapted from refs. [11,12,18]. Improved therapeutic outcomes are achieved with high RBE and low OER values.
The majority of DNA damage by either high or low LET radiation is thought to arise indirectly through production of reactive oxygen species (ROS). Oxygenated tissues are more sensitive to low LET radiation, reflecting the requirement for ROS production to damage DNA. Unfortunately, many tumors are hypoxic, or have hypoxic regions, and these show significant radioresistance to low LET radiation. One measure of this resistance is the “oxygen enhancement ratio” (OER), which is the ratio of doses required to produce the same biological effect with a given type of radiation in the presence or absence of oxygen [17]. For both photon and proton radiation, the OER is ~3, meaning that cell killing is ~3-fold greater in normoxic vs hypoxic conditions. Importantly, carbon ions show a much lower OER (Fig. 2b), thus even hypoxic tumors show significant sensitivity to high LET carbon ion radiation, most likely reflecting the larger mass of carbon ions that achieve dense ionization even in hypoxic conditions, resulting in more clustered (difficult to repair) DNA damage [18,19].
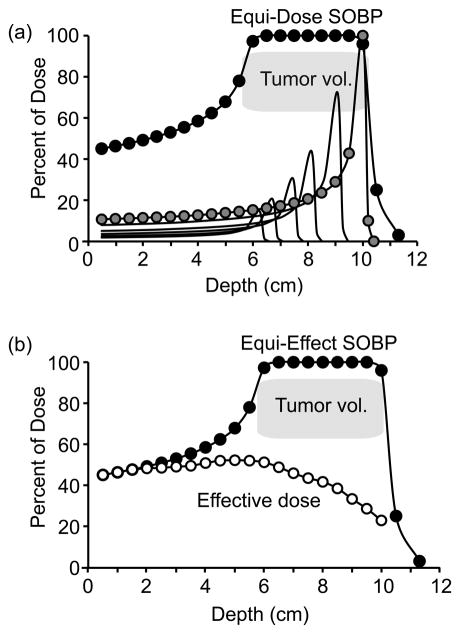
The Bragg peak for monoenergetic proton and carbon beams is narrow and well focused. However tumors can be extensive with irregular dimensions. A “spread out Bragg peak” (SOBP) is required to insure that heavy ions stop in all regions of the tumor. As mentioned earlier, the location of the Bragg peak can be adjusted by adjusting the energy of the incident beam. The Bragg peak of a single carbon beam, and a SOBP that creates an equal dose in the designated tumor volume, are shown in Fig. 3a. Note that the dose to the distal end of the tumor is delivered exclusively by stopping particles. The doses to proximal regions of the tumor have a successively reduced contribution from smaller peaks (lower incident energy) to accommodate for the accumulated doses from particles passing through and stopping at greater distances. Thus, even though the absorbed dose is uniform, there is a changing distribution of energies, and therefore a changing distribution of LET, as a function of depth in the tumor. The objective for successful cancer therapy is to insure uniform killing throughout the treatment volume. However as described earlier, the RBE for cell killing increases with LET. Thus the dose in the SOBP should not be uniform, but is adjusted to account for RBE. An example of an equi-effect SOBP, where doses are varied to account for changing LET in the tumor volume, is shown in Fig. 3b.
Fig. 3.
Equi-dose and equi-effect SOBP dose distributions. (a) A 4 cm equi-dose SOBP beginning at a depth of 6 cm incident carbon ions (solid circles). The SOBP is created from multiple beams with variable energy (shown by the set of thin lines); the Bragg peak for the highest incident energy ions is shown with gray circles. The SOBP is designed to deliver equal dose across the tumor volume. (b) A 4 cm equi-effect SOBP beginning at a depth of 6 cm for incident carbon ions. The dose to achieve equal cell killing in the SOBP is reduced at distal sites because LET and RBE increase with depth.
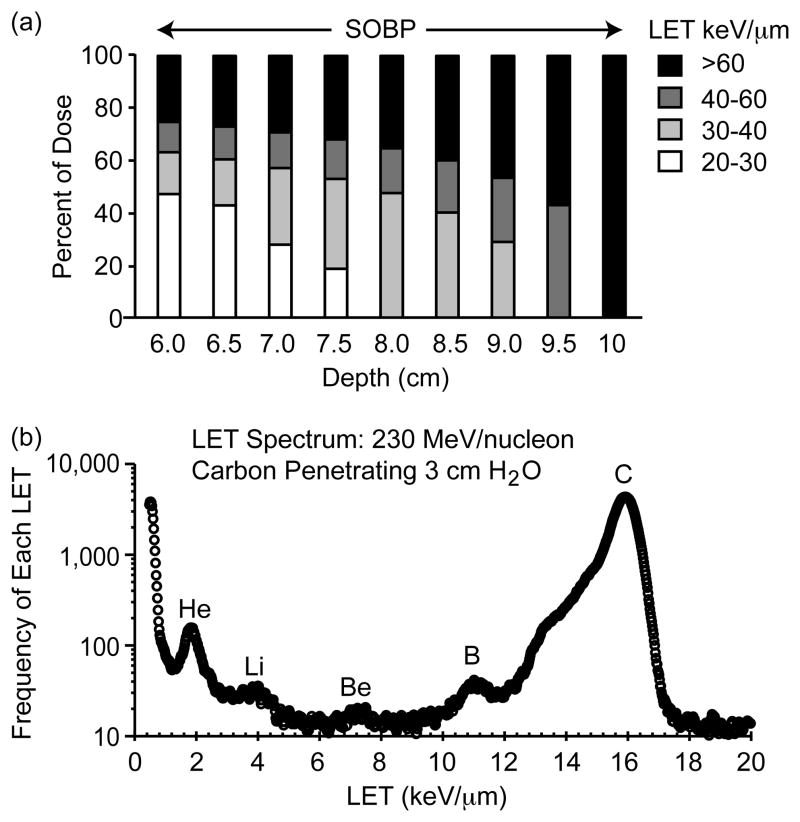
The LET changes within the SOBP because different incident energies are used to create a distribution of Bragg peaks (Fig. 3a), and because of particle fragmentation. As shown in Fig. 4a, the distal region of the tumor receives a dose that is almost exclusively high LET, but the dose to the proximal region is mostly low LET. Protons and carbon ions are classified as hadrons and are thus capable of nuclear interactions. When HZE particles penetrate tissue, some particles interact with nuclei, causing fragmentation of the incident particle. These projectile fragments have the same velocity as the original particle but reduced mass and charge. This results in a mixture of particles with different LET passing through the tumor. The LET distribution of carbon and fragments at a depth of 3 cm in water for incident carbon with an energy of 230 MeV/nucleon is shown in Fig. 4b. In this instance, about 25% of the particles are fragments with reduced LET. These fragments must be accounted for because they contribute a low LET dose to the tumor, and because low LET particles penetrate farther into tissue and could contribute to a dose to normal tissue beyond the Bragg peak of the stopping carbon ions.
Fig. 4.
Changing LET spectrum of carbon ion radiation. (a) Distribution of LET within a 4 cm equi-dose SOBP for incident carbon ions. (b) Distribution of fragments at a depth of 3 cm in water from an incident carbon ion beam at 230 MeV/nucleon. The data are plotted as the frequency of particles as a function of LET. The main peak is from carbon ions after penetrating 3 cm of water. The lower LET particles are fragments with certain ion peaks identified.
Because hadron beams are so tightly focused, accurate beam delivery is essential. The particle fragments produced by carbon ions allow precise monitoring of doses to tissues by using in-beam positron emission tomography (PET) which detects β+ emitting fragments (e.g., 10C, 11C, and 15O). This is a well-established technique for carbon ions, and recent studies suggest this technique may also be feasible for photon and proton beams, although the need for precision dose delivery is certainly greater with the more focused carbon ion and proton beams [20–22].
4. Radiosensitizers, radioprotectors, and risks of radiation exposure
Several approaches have proven effective in sensitizing cells to the killing effects of radiation, including hyperthermia, small molecule drugs or genetic approaches to inhibit DNA repair, and increasing oxygen [23–25]. Cells are sensitized to low LET IR by increased oxygen because of the marked OER displayed by these radiations. The lower OER of high LET IR indicates that increasing oxygen will provide limited sensitization to carbon ion radiation. DNA repair systems are important targets for radiosensitization, with significant attention paid to non-homologous end-joining since this pathway is responsible for repairing the majority of DNA double-strand breaks (DSBs), the major cytotoxic lesion induced by IR. Other pathways under investigation are homologous recombination and base excision repair [26,27]. Increasing attention is being paid to radiosensitization by targeting the broader DNA damage response, including checkpoint proteins [28]. DNA repair and checkpoint systems can be inactivated by genetic approaches, such as siRNA knockdown of critical repair proteins, or by using small molecule inhibitors of these proteins. In contrast, radioprotection of normal tissues can be afforded by the use of free radical scavengers or by enhancing repair capacity [29]. Although these approaches have proven effective in the laboratory, they have found limited utility in the clinic because of both normal and tumor cells rely on repair and checkpoint systems for survival hence inhibiting these systems does not necessarily improve therapeutic index. In theory radiosensitizers specifically targeted to tumor cells (or radioprotectors to normal tissue) would enhance the therapeutic index, but efficient delivery and/or targeting appropriate cells presents significant challenges. Such approaches may be less important for hadron RT because hadron radiation can be delivered to a target volume with high accuracy, and in the case of carbon ions, the complex damage they produce is highly lethal and further sensitization by drug or genetic approaches may not be necessary.
There are known risks associated with photon and hadron radiation. Damage from both low and high LET radiation causes mutations, which could produce secondary tumors. This risk is relatively low and well within acceptable risk-benefit ratio for photon and proton RT, although there is increased concern with younger patients. It is too early to evaluate the risk of secondary cancer from carbon ion RT, but concerns have been raised [19]. Because the risk of secondary cancer is restricted to the exposed tissue, the superior dose distributions of protons and carbon ions help to minimize the volume of at-risk tissue. The immediate benefits of RT typically outweigh these risks, although there remains significant concern for pediatric patients due to their longer lifespan potential and the increased chance of developing cancer when growing tissues are irradiated.
Irradiated cells may carry an increased mutation load that could drive transformation of normal cells or progression of surviving tumor cells leading to aggressive local recurrence and/or metastasis. The mutagenic risks for photon and proton RT appear to be low, but there is increased concern about mutagenesis induced by heavy hadron radiation (carbon, space radiation) because complex DNA damage is thought to be more mutagenic [19]. Substantial data support this notion in model systems (e.g., repair of clustered damage in oligonucleotides or plasmids) [19], but results in tissue culture and animal models are not easily interpreted, as discussed in the next section.
Negative sequelae from radiation exposure that occur more quickly include tissue damage (“late tissue injury”) such as radiation-induced fibrosis and necrosis [30], and tumor repopulation [31] leading to local recurrence. Studies of patients treated with current carbon ion RT protocols suggest that serious late tissue injury occurs no more often, and usually less often, than observed with photon or proton RT (see Hadron Radiation for Cancer Therapy, below). Further research is required to identify potential differences in the frequencies of negative sequelae after treatment with each type of radiation.
5. Hadron Radiation and Mutagenesis
The densely ionizing (high LET) radiation achieved with carbon and other high-mass particles is thought to create greater amounts of clustered DNA damage, which poses significant challenges for cellular DNA repair systems. Clustered DNA damage produced by a single radiation track through a cell may comprise multiple single-strand breaks and DSBs, abasic sites and other forms of base damage within one or two turns of the DNA double helix [18]. When normal human fibroblasts were exposed to hadron radiation with different ions such as oxygen, iron, or silicon, iron ions produced more persistent DSBs, minimal repair, and decreased survival suggesting that the higher LET of the heavier ions generates more clustered DNA damage that is difficult to repair [32,33]. The hyperthermal secondary fragments that are generated as the particle traverses the cell also contribute to clustered DNA damage because they can scatter from 5–10 nm of their formation site and cause ancillary DNA damage to the opposite phosphate-sugar backbone [34].
At the molecular level, complex damage can be inferred from the size of DNA deletions resulting from radiation exposure. Sequence comparison of deletions in gpt delta transgenic mice irradiated with 10 Gy X-rays, γ-rays or heavy ions showed that heavy ion treatment often produced deletions in excess of 1000 bp while X-rays and γ-rays typically yielded deletions of less than 100 bp [35]. These authors suggested that most photon-induced mutations arose through indirect effects such as ROS generation, whereas heavy ions induced mutations via more direct ionization of DNA. A recent study comparing γ-ray and carbon ion irradiation of plasmid DNA in the presence or absence of the free-radical scavenger mannitol supports this idea. Mannitol abrogated the linearization of supercoiled plasmid DNA by γ-rays, but even high concentrations of mannitol had no effect on plasmid linearization by carbon ions [36]. This in vitro study also supports the idea that DNA damage created by heavy ions is not strongly influenced by environmental factors.
Analysis of repair and mutagenesis in engineered oligonucleotide or plasmid substrates demonstrates the increased mutability of clustered damage [19], but studies of mutagenesis at chromosomal loci in cells and animals are not easy to interpret. For example, mutation induction in the autosomal TK and X-linked HPRT loci shows differential responses to LET ranging from 32–190 keV/μm, with TK mutagenesis increasing up to 61 keV/μm but decreasing at higher LET, whereas mutability at HPRT plateaus above 61 keV/μm [37]. Moreover, mice irradiated with equivalent doses of photons and heavy (Fe) ions showed a ~50-fold higher rate of liver cancer with heavy ions, but no difference in leukemia induction [38]. Many factors are likely to contribute to differential mutability, and susceptibility to radiation-induced cancer, of cells and tissues to low and high LET radiation, including genetic background (i.e., repair capacity and DNA damage checkpoint status), fractions of proliferating cells vs post-mitotic cells, proliferation rates and cell cycle distributions, dose and dose-rate effects, and gene locus and tissue-specific effects.
Although cells typically arrest in the cell cycle after radiation exposure, they may enter mitosis with as many as 10–20 DSBs [39]. Cells that accumulate mutations and manifest a negligible G2/M checkpoint may eventually promote carcinogenesis [40] and contribute to tumor repopulation [31]. Chromosome aberrations such as complex rearrangements, and changes in chromosome numbers, can occur after exposure to HZE radiation in space or charged particle beams from accelerators [41,42]. Cytogenetic dosimetry is being developed as a diagnostic tool to assay chromosome damage in space travelers [43–46] and will likely also prove useful in radiotherapy clinics.
6. Gene expression studies
Genome-wide transcriptional changes in response to photon radiation have been widely studied [47], but few comparable studies with heavy ion radiation have been reported. Two studies examined gene expression changes in response to carbon ion treatment. Vascular endothelial growth factor (VEGF) mRNA levels were monitored and found to be induced in RERF-LC-AI lung squamous carcinoma cells by a maximum of 2.8-fold in a LET dose–dependent manner after treatment with 15 Gy of high LET (13.3, 50 and 90 keV/μm) carbon ion radiation [48]. Using high-coverage expression profiling (HiCEP), Fujimori and co-workers found the abnormal spindle-like microcephaly associated gene (ASPM) was downregulated in human diploid fibroblasts after exposure to 2 Gy of high LET (70 keV/μm) carbon ions [49]. Further gene expression studies should prove useful in identifying potential gene targets that may be manipulated to sensitize tumor cells, or protect normal cells, during carbon ion radiotherapy.
7. Hadron Radiation for Cancer Therapy
Hadron-particle radiotherapy using protons and heavy ions has advantages for treating cancer patients with tumors that are difficult if not impossible to treat with conventional photon radiotherapy. Superior dose distribution from charged-particle beams can provide improved targeting of tumors with preferential localized cell killing that results in reduced ancillary damage to surrounding tissues. Robert R. Wilson recognized the potential of heavy charged particles and first proposed the use of fast protons for radiotherapy in 1946 [50]. The first treatments using protons and helium ions began in 1954 and 1957 respectively at the Lawrence Berkeley National Laboratory [51]. A total of 78,275 patients had received hadron-particle treatment by the end of 2009. Proton treatment accounts for the majority of the patients treated (67,097), with helium (2054) carbon (7151), other ions (873) and pions (1100) making up the rest [52]. Currently there are 29 facilities in operation worldwide that are dedicated to proton treatment [53] but only five facilities are treating patients with carbon ions, three of which are in Japan, including the Heavy Ion Medical Accelerator in Chiba (HIMAC) at the National Institute of Radiological Sciences (NIRS), which first began patient treatment in 1994, Hyogo Ion Beam Medical Center, and Gunma University Heavy-Ion Medical Center. The other facilities include the Heidelberg Ion Beam Therapy Centre, which began treating patients in 2009, and the Institute of Modern Physics, in which only low energy carbon ion beams are available.
Carbon ion therapy often involves hypofractionated treatment regimens, with roughly half the number of fractions of standard photon-based radiotherapy. Carbon ion regimens that average 12.5 fractions over three weeks (but may have as few as 1–2 fractions for lung or liver cancer) are currently in use or are being implemented because of the superior dose distribution and enhanced RBE of carbon ions. Carbon ion RT is gaining acceptance as more patient data are collected. There is a strong rationale for initiating randomized clinical trials to directly compare proton and carbon ion RT [7], yet none have been completed, although one trial was recently initiated at the Heidelberg facility [54]. Nearly 50 carbon ion RT Phase I/II and Phase II protocol studies have been performed at the NIRS since 1994 on a wide range of tumor types including head/neck, lung, liver, prostate, bone and soft tissue, uterine, brain, esophageal, pancreas, rectum, eye (melanoma), and lacrimal gland. The lack of randomized clinical trials prevents direct comparisons among photon, proton, and carbon ion therapies, but clinical outcomes data including local tumor control, survival, and late injury rates (controlled for tumor stage) reveals interesting trends, as summarized in Tables 1–4. These data indicate that compared to photon or proton RT, carbon ion RT typically gives superior local tumor control with equal or lower rates of negative sequelae. Of particular note, carbon ion RT is achieving >90% five-year local control and from 33 to >90% five-year survival with prostate, pancreatic adenocarcinoma, and post-operative pelvic recurrent rectal cancer, sites where photon treatment is much less effective [55]. Additional comparative analyses of photon and hadron RT clinical outcomes are described in Suit et al. [7].
Table 1.
Adenoid Cystic Carcinoma - Head/Neck
| Treatment | No. of Cases | 5 year Local Control (%) | 5 year Survival (%) | Late Injury ≥G3 (%) | References |
|---|---|---|---|---|---|
| Photon | 10 | 27 | 25 | -- | [63] |
| Photon | 101 | 56 | 57 | 6 | [64] |
| Protona | 23 | 93 | 77 | 17.4 | [65] |
| Carbon | 134 | 81 | 72 | 0 (G2 <3%) | b |
10 cases included surgical resection
Reported at the Joint NIRS-Karolinska Institute Symposium on Ion Radiation Sciences, Sept. 2010.
Table 4.
Non-Small Cell Lung Cancer
| Treatment | No. T1/T2 Cases | T1 5 year Local Control (%) | T2 5 year Local Control (%) | T1 5 year Survival (%) | T2 5 year Survival (%) | References |
|---|---|---|---|---|---|---|
| Protona | 37/9 | 89 | 39 | 70 | 16 | [71] |
| Carbonb | 29/21 | 96 | 93 | 55 | 43 | [72,73] |
| Carbonc | 42/37 | 98 | 80 | 62 | 25 | [74] |
Proton fractions = 7–32 (median of 22), with 49–93 Gy (median 76 Gy) delivered over 10–66 weeks (median 41 weeks).
Carbon ion fractions = 9, with 72 GyE delivered over 3 weeks.
Carbon ion fractions = 4, with 52.8–60 GyE delivered over 1 week.
As of July 2010 another 23 facilities for proton and/or carbon treatment are either proposed or under construction [56]. Currently, the biological responses of tumors and normal tissue to heavy ion radiation are being studied at facilities in Japan, Brookhaven National Laboratory, Gesellschaft für Schwerionenforschung in Darmstadt, Germany, and other locations. For additional information about the technical and clinical aspects of hadron radiotherapy, the reader is directed to several excellent reports and books [5,9,10,51,57–62].
8. Future directions
Heavy ion radiobiology research has aided design and implementation of more effective cancer treatments and improved understanding of some of the inherent dangers associated with space travel. Further work on several fronts remains. Comparative studies of the different radiation types will provide new biological insights that will drive translational studies aimed at customizing radiation treatment to tumor type and grade. Much remains to be learned about DNA damage responses including radiation-induced protein modifications (such as histone methylation and acetylation) and gene expression changes with various radiation types. New technologies such as carbon ion pencil-beam technology that allows tumors to be “painted” with thin beams of heavy ions will usher in a new era of hadron RT. These and other novel treatment strategies will be informed by studies at the interface between radiation physics and biology, such as the biological effects of particle fragments and mixed LET radiation characteristic of heavy ion radiation-tissue interactions.
IR, like genotoxic chemicals and topoisomerase inhibitors, is very effective at killing rapidly growing cells comprising the bulk of solid tumors, due to the high sensitivity of cells to DNA damage during DNA replication. However, to prevent tumor recurrence, a key target may be slow-growing cancer stem cells, as these cells may repair DNA damage prior to reentering the cell cycle and initiating DNA synthesis. An important question, therefore, is whether DNA damage created by high LET radiation persists long enough to inactivate cancer stem cells. Carbon ions may be particularly effective in killing cancer stem cells for another reason: these cells may be protected from low LET radiation if they occupy hypoxic compartments, but hypoxia should not protect them from high LET carbon ion radiation.
It has long been known that dying tumor cells following radiation stimulate rapid proliferation of surviving cells, and the Li laboratory recently demonstrated that the caspase 3-apoptosis pathway is required for this accelerated re-population effect after low LET radiation [31]. It remains to be seen whether similar mechanisms function in cells treated with hadron radiation. Radiation is an excellent tool for local tumor control, but it is not effective once tumors have metastasized to distant sites. In these situations, systemic chemotherapy is required, but patients tolerate radiation far better than chemotherapy, and improved tumor imaging to detect micro-metastases may allow radiation to be used effectively to treat even the most challenging, late-stage cancer cases.
Table 2.
Chordoma – Skull Base and Paracervical Spine
| Treatment | No. of Cases | 5 year Local Control (%) | 10 year Local Control (%) | References |
|---|---|---|---|---|
| Photon | 24 | 23 | 15 | [66] |
| Proton | 169 | 73 | 54 | [67] |
| Carbona | 96 | 70 | Not reported | [68] |
| Carbonb | 39 | 82 | 82 | Tsujii, unpublished results |
| Carbonc | 29 | 95 | ND | Tsujii, unpublished results |
60 Gy equivalent (GyE) median dose. For carbon ions, GyE is 3-fold higher than actual dose (Gy) because it takes into account the 3-fold higher RBE.
48–60.8 GyE median dose
60.8 Gy median dose
Table 3.
Retroperitoneal Sarcoma
| Treatment | No. of Cases | 5 year Local Control (%) | 5 year Survival (%) | References |
|---|---|---|---|---|
| Photona | 67 | 40 | 58 | [69] |
| Carbonb | 27 | 65 | 69 | [70] |
Maximal surgical resection plus intraoperative electron beam RT (too few patients have been treated with proton RT alone).
Carbon ion RT only
Acknowledgments
We thank T. Kato, T. Kamada, P. Jeggo, R. Okayasu, N. Matsufuji, S. Bailey, H. Liber, C. Li, and A. Fujimori for thoughtful discussions. Research in the Nickoloff lab is supported by NIH grant R01 GM084020. Research in the Borak laboratory is supported by the NSF (grant 154-5174/SPO68027), Nanosonic, Inc. (contract N-A85P), and NASA (NSBRI 5600283624 Project RE01701 and NCC 9-58-10 Project RE01301).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muller HJ. Artificial transmutation of the gene. Science. 1927;66:84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford E. Atomic Projectiles and Their Collisions with Light Atoms. Science. 1919;50:467–473. doi: 10.1126/science.50.1299.467. [DOI] [PubMed] [Google Scholar]
- 3.Weber U, Kraft G. Comparison of carbon ions versus protons. Cancer J. 2009;15:325–332. doi: 10.1097/PPO.0b013e3181b01935. [DOI] [PubMed] [Google Scholar]
- 4.Bragg WH. On the ionization of various gases by the alpha particles of radium. No. 2. Proc Phys Soc London. 1907;20:0523–0550. [Google Scholar]
- 5.DeLaney T, Kooy HM. Proton and Charged Particle Radiotherapy. Lippincott, Williams and Wilkins; Philadelphia: 2007. [Google Scholar]
- 6.Lomax AJ. Charged particle therapy: the physics of interaction. Cancer J. 2009;15:285–291. doi: 10.1097/PPO.0b013e3181af5cc7. [DOI] [PubMed] [Google Scholar]
- 7.Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, Niemierko A, Hall E, Flanz J, Hallman J, Trofimov A. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010;95:3–22. doi: 10.1016/j.radonc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Akiko U, Koichi A, Sachiko K, Yoshiya F, Yoshitaka M, Nobuhiko T, Ryoichi H, Masahiko W, Michael S, Thilo E, Peter P. Comparison of biological effectiveness of carbon-ion beams in Japan and Germany. Int J Radiat Oncol Biol Phys. 2009;73:1545–1551. doi: 10.1016/j.ijrobp.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Fokas E, Kraft G, An H, Engenhart-Cabillic R. Ion beam radiobiology and cancer: Time to update ourselves. Biochim Biophys Acta. 2009;1796:216–229. doi: 10.1016/j.bbcan.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Schulz-Ertner D, Jakel O, Schlegel W. Radiation therapy with charged particles. Semin Radiat Oncol. 2006;16:249–259. doi: 10.1016/j.semradonc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Paganetti H. Significance and implementation of RBE variations in proton beam therapy. Technol Cancer Res Treat. 2003;2:413–426. doi: 10.1177/153303460300200506. [DOI] [PubMed] [Google Scholar]
- 12.Weyrather WK, Kraft G. RBE of carbon ions: experimental data and the strategy of RBE calculation for treatment planning. Radiother Oncol. 2004;73(Suppl 2):S161–169. doi: 10.1016/s0167-8140(04)80041-0. [DOI] [PubMed] [Google Scholar]
- 13.Hodgkins PS, O'Neil P, Stevens D, Fairman MP. The severity of alpha-particle-induced DNA damage is revealed by exposure to cell-free extracts. Radiat Res. 1996;146:660–667. [PubMed] [Google Scholar]
- 14.Okayasu R, Okada M, Okabe A, Noguchi M, Takakura K, Takahashi S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat Res. 2006;165:59–67. doi: 10.1667/rr3489.1. [DOI] [PubMed] [Google Scholar]
- 15.Tsao D, Kalogerinis P, Tabrizi I, Dingfelder M, Stewart RD, Georgakilas AG. Induction and processing of oxidative clustered DNA lesions in 56Fe-ion-irradiated human monocytes. Radiat Res. 2007;168:87–97. doi: 10.1667/RR0865.1. [DOI] [PubMed] [Google Scholar]
- 16.Georgakilas AG, Bennett PV, Wilson DM, 3rd, Sutherland BM. Processing of bistranded abasic DNA clusters in gamma-irradiated human hematopoietic cells. Nucleic Acids Res. 2004;32:5609–5620. doi: 10.1093/nar/gkh871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weyrather WK, Debus J. Particle beams for cancer therapy. Clin Oncol. 2003;15:S23–28. doi: 10.1053/clon.2002.0185. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland BM, Bennett PV, Schenk H, Sidorkina O, Laval J, Trunk J, Monteleone D, Sutherland J. Clustered DNA damages induced by high and low LET radiation, including heavy ions. Phys Med. 2001;17(Suppl 1):202–204. [PubMed] [Google Scholar]
- 19.Hada M, Georgakilas AG. Formation of clustered DNA damage after high-LET irradiation: a review. J Radiat Res (Tokyo) 2008;49:203–210. doi: 10.1269/jrr.07123. [DOI] [PubMed] [Google Scholar]
- 20.Parodi K, Enghardt W, Haberer T. In-beam PET measurements of beta+ radioactivity induced by proton beams. Phys Med Biol. 2002;47:21–36. doi: 10.1088/0031-9155/47/1/302. [DOI] [PubMed] [Google Scholar]
- 21.Kluge T, Mockel D, Pawelke J, Enghardt W. First in-beam PET measurement of beta+ radioactivity induced by hard photon beams. Phys Med Biol. 2007;52:N467–473. doi: 10.1088/0031-9155/52/20/N01. [DOI] [PubMed] [Google Scholar]
- 22.Ponisch F, Parodi K, Hasch BG, Enghardt W. The modelling of positron emitter production and PET imaging during carbon ion therapy. Phys Med Biol. 2004;49:5217–5232. doi: 10.1088/0031-9155/49/23/002. [DOI] [PubMed] [Google Scholar]
- 23.Iliakis G, Wu W, Wang M. DNA double strand break repair inhibition as a cause of heat radiosensitization: re-evaluation considering backup pathways of NHEJ. Int J Hyperthermia. 2008;24:17–29. doi: 10.1080/02656730701784782. [DOI] [PubMed] [Google Scholar]
- 24.Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;7:492–508. doi: 10.1634/theoncologist.7-6-492. [DOI] [PubMed] [Google Scholar]
- 25.Vallerga AK, Zarling DA, Kinsella TJ. New radiosensitizing regimens, drugs, prodrugs, and candidates. Clin Adv Hematol Oncol. 2004;2:793–805. [PubMed] [Google Scholar]
- 26.Barker CA, Powell SN. Enhancing radiotherapy through a greater understanding of homologous recombination. Semin Radiat Oncol. 2010;20:267–273. doi: 10.1016/j.semradonc.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vens C, Begg AC. Targeting base excision repair as a sensitization strategy in radiotherapy. Semin Radiat Oncol. 2010;20:241–249. doi: 10.1016/j.semradonc.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Luo Y, Leverson JD. New opportunities in chemosensitization and radiosensitization: modulating the DNA-damage response. Expert Rev Anticancer Ther. 2005;5:333–342. doi: 10.1586/14737140.5.2.333. [DOI] [PubMed] [Google Scholar]
- 29.Greenberger JS. Radioprotection. In Vivo. 2009;23:323–336. [PMC free article] [PubMed] [Google Scholar]
- 30.Delanian S, Lefaix JL. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol. 2007;17:99–107. doi: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, Li CY. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asaithamby A, Uematsu N, Chatterjee A, Story MD, Burma S, Chen DJ. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiat Res. 2008;169:437–446. doi: 10.1667/RR1165.1. [DOI] [PubMed] [Google Scholar]
- 33.Sekine E, Okada M, Matsufuji N, Yu D, Furusawa Y, Okayasu R. High LET heavy ion radiation induces lower numbers of initial chromosome breaks with minimal repair than low LET radiation in normal human cells. Mutat Res. 2008;652:95–101. doi: 10.1016/j.mrgentox.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Deng Z, Bald I, Illenberger E, Huels MA. Reactive scattering damage to DNA components by hyperthermal secondary ions. Phys Rev Lett. 2006;96:243203. doi: 10.1103/PhysRevLett.96.243203. [DOI] [PubMed] [Google Scholar]
- 35.Masumura K-i, Kuniya K, Kurobe T, Fukuoka M, Yatagai F, Nohmi T. Heavy-ion-induced mutations in the gpt delta transgenic mouse: Comparison of mutation spectra induced by heavy-ion, X-ray, and γ-ray radiation. Envir Mol Mutagen. 2002;40:207–215. doi: 10.1002/em.10108. [DOI] [PubMed] [Google Scholar]
- 36.Dang HM, van Goethem MJ, van der Graaf ER, Brandenburg S, Hoekstra R, Schlathölter T. Plasmid DNA damage by heavy ions at spread-out Bragg peak energies. Eur Phys J D. 2010;60:51–58. [Google Scholar]
- 37.Kronenberg A. Mutation induction in human lymphoid cells by energetic heavy ions. Adv Space Res. 1994;14:339–346. doi: 10.1016/0273-1177(94)90486-3. [DOI] [PubMed] [Google Scholar]
- 38.Weil MM, Bedford JS, Bielefeldt-Ohmann H, Ray FA, Genik PC, Ehrhart EJ, Fallgren CM, Hailu F, Battaglia CL, Charles B, Callan MA, Ullrich RL. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon (56)Fe ions. Radiat Res. 2009;172:213–219. doi: 10.1667/RR1648.1. [DOI] [PubMed] [Google Scholar]
- 39.Deckbar D, Birraux J, Krempler A, Tchouandong L, Beucher A, Walker S, Stiff T, Jeggo P, Löbrich M. Chromosome breakage after G2 checkpoint release. J Cell Biol. 2007;176:749–755. doi: 10.1083/jcb.200612047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7:861–869. doi: 10.1038/nrc2248. [DOI] [PubMed] [Google Scholar]
- 41.Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nat Rev Cancer. 2008;8:465–472. doi: 10.1038/nrc2391. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki M, Kase Y, Yamaguchi H, Kanai T, Ando K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int J Radiat Oncol Biol Phys. 2000;48:241–250. doi: 10.1016/s0360-3016(00)00568-x. [DOI] [PubMed] [Google Scholar]
- 43.Durante M, George K, Yang TC. Biodosimetry of ionizing radiation by selective painting of prematurely condensed chromosomes in human lymphocytes. Radiat Res. 1997;148:S45–S50. [PubMed] [Google Scholar]
- 44.Edwards AA. The use of chromosomal aberrations in human lymphocytes for biological dosimetry. Radiat Res. 1997;148:S39–S44. [PubMed] [Google Scholar]
- 45.Lucas JN. Dose reconstruction for individuals exposed to ionizing radiation using chromosome painting. Radiat Res. 1997;148:S33–S38. [PubMed] [Google Scholar]
- 46.Wu H, Durante M, George K, Yang TC. Induction of chromosome aberrations in human cells by charged particles. Radiat Res. 1997;148:S102–S107. [PubMed] [Google Scholar]
- 47.Amundson SA. Functional genomics in radiation biology: a gateway to cellular systems-level studies. Radiat Environ Biophys. 2008;47:25–31. doi: 10.1007/s00411-007-0140-1. [DOI] [PubMed] [Google Scholar]
- 48.Ando S, Nojima K, Ishihara H, Suzuki M, Ando M, Majima H, Ando K, Kuriyama T. Induction by carbon-ion irradiation of the expression of vascular endothelial growth factor in lung carcinoma cells. Int J Radiat Biol. 2000;76:1121–1127. doi: 10.1080/09553000050111596. [DOI] [PubMed] [Google Scholar]
- 49.Fujimori A, Yaoi T, Ogi H, Wang B, Suetomi K, Sekine E, Yu D, Kato T, Takahashi S, Okayasu R, Itoh K, Fushiki S. Ionizing radiation downregulates ASPM, a gene responsible for microcephaly in humans. Biochem Biophys Res Comm. 2008;369:953–957. doi: 10.1016/j.bbrc.2008.02.149. [DOI] [PubMed] [Google Scholar]
- 50.Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 51.Jereczek-Fossa BA, Krengli M, Orecchia R. Particle beam radiotherapy for head and neck tumors: radiobiological basis and clinical experience. Head Neck. 2006;28:750–760. doi: 10.1002/hed.20448. [DOI] [PubMed] [Google Scholar]
- 52.Jermann M. Hadron Therapy Patient Statistics, Particle Therapy Co-Operative Group (PTCOG) 2010. [Google Scholar]
- 53.Jermann M. Particle therapy facilities in operation (incl patient statistics), Particle Therapy CoOperative Group (PTCOG) 2010. [Google Scholar]
- 54.Nikoghosyan AV, Karapanagiotou-Schenkel I, Münter MW, Jensen AD, Combs SE, Debus J. Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer. 2010;10:607. doi: 10.1186/1471-2407-10-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okada T, Kamada T, Tsuji H, Mizoe JE, Baba M, Kato S, Yamada S, Sugahara S, Yasuda S, Yamamoto N, Imai R, Hasegawa A, Imada H, Kiyohara H, Jingu K, Shinoto M, Tsujii H. Carbon ion radiotherapy: clinical experiences at National Institute of Radiological Science (NIRS) J Radiat Res (Tokyo) 2010;51:355–364. doi: 10.1269/jrr.10016. [DOI] [PubMed] [Google Scholar]
- 56.PTCOG. Particle therapy facilities in a planning stage or under construction, Particle Therapy CoOperative Group (PTCOG) 2010. [Google Scholar]
- 57.Goitein M. Radiation Oncology: A Physicist's-Eye View. Springer Science+Business Media; LLC, New York: 2007. [Google Scholar]
- 58.Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, Kato H, Yamada S, Morita S, Yoshikawa K, Kandatsu S, Tateishi A. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466–4471. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 59.Blakely EA, Kronenberg A. Heavy-ion radiobiology: new approaches to delineate mechanisms underlying enhanced biological effectiveness. Radiat Res. 1998;150:S126–S145. [PubMed] [Google Scholar]
- 60.Tsujii H, Kamada T, Baba M, Tsuji H, Kato H, Kato S, Yamada S, Yasuda S, Yanagi T, Kato H, Hara R, Yamamoto N, Mizoe J. Clinical advantages of carbon-ion radiotherapy. New J Phys. 2008;10:1–16. [Google Scholar]
- 61.Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7:37–43. doi: 10.1038/nrclinonc.2009.183. [DOI] [PubMed] [Google Scholar]
- 62.Brower V. Carbon ion therapy to debut in Europe. J Natl Canc Inst. 2009;101:74–76. doi: 10.1093/jnci/djn496. [DOI] [PubMed] [Google Scholar]
- 63.Iseli TA, Karnell LH, Graham SM, Funk GF, Buatti JM, Gupta AK, Robinson RA, Hoffman HT. Role of radiotherapy in adenoid cystic carcinoma of the head and neck. J Laryngol Otol. 2009;123:1137–1144. doi: 10.1017/S0022215109990338. [DOI] [PubMed] [Google Scholar]
- 64.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–162. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 65.Pommier P, Liebsch NJ, Deschler DG, Lin DT, McIntyre JF, Barker FG, 2nd, Adams JA, Lopes VV, Varvares M, Loeffler JS, Chan AW. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:1242–1249. doi: 10.1001/archotol.132.11.1242. [DOI] [PubMed] [Google Scholar]
- 66.Catton C, O'Sullivan B, Bell R, Laperriere N, Cummings B, Fornasier V, Wunder J. Chordoma: long-term follow-up after radical photon irradiation. Radiother Oncol. 1996;41:67–72. doi: 10.1016/s0167-8140(96)91805-8. [DOI] [PubMed] [Google Scholar]
- 67.Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175(Suppl 2):57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- 68.Schulz-Ertner D, Karger CP, Feuerhake A, Nikoghosyan A, Combs SE, Jakel O, Edler L, Scholz M, Debus J. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. 2007;68:449–457. doi: 10.1016/j.ijrobp.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 69.Krempien R, Roeder F, Oertel S, Weitz J, Hensley FW, Timke C, Funk A, Lindel K, Harms W, Buchler MW, Debus J, Treiber M. Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2006;65:773–779. doi: 10.1016/j.ijrobp.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 70.Serizawa I, Kagei K, Kamada T, Imai R, Sugahara S, Okada T, Tsuji H, Ito H, Tsujii H. Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2009;75:1105–1110. doi: 10.1016/j.ijrobp.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 71.Shioyama Y, Tokuuye K, Okumura T, Kagei K, Sugahara S, Ohara K, Akine Y, Ishikawa S, Satoh H, Sekizawa K. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;56:7–13. doi: 10.1016/s0360-3016(02)04416-4. [DOI] [PubMed] [Google Scholar]
- 72.Miyamoto T, Yamamoto N, Nishimura H, Koto M, Tsujii H, Mizoe JE, Kamada T, Kato H, Yamada S, Morita S, Yoshikawa K, Kandatsu S, Fujisawa T. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. 2003;66:127–140. doi: 10.1016/s0167-8140(02)00367-5. [DOI] [PubMed] [Google Scholar]
- 73.Miyamoto T, Baba M, Yamamoto N, Koto M, Sugawara T, Yashiro T, Kadono K, Ezawa H, Tsujii H, Mizoe JE, Yoshikawa K, Kandatsu S, Fujisawa T. Curative treatment of Stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007;67:750–758. doi: 10.1016/j.ijrobp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 74.Miyamoto T, Baba M, Sugane T, Nakajima M, Yashiro T, Kagei K, Hirasawa N, Sugawara T, Yamamoto N, Koto M, Ezawa H, Kadono K, Tsujii H, Mizoe JE, Yoshikawa K, Kandatsu S, Fujisawa T. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. 2007;2:916–926. doi: 10.1097/JTO.0b013e3181560a68. [DOI] [PubMed] [Google Scholar]