Figure 1. The coeliac lesion in the proximal small intestine.
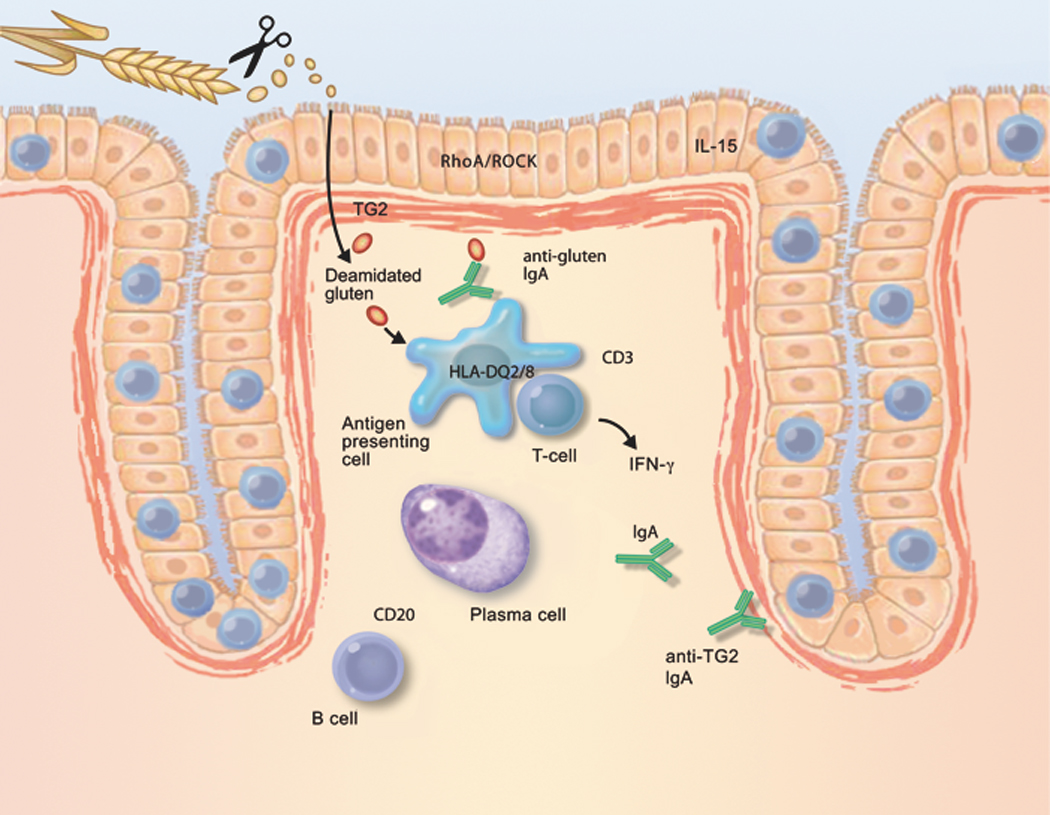
Schematic depiction of factors that contribute to the development of coeliac disease, and that could be novel therapeutic targets. Long, proline-rich fragments of gluten survive digestion by luminal and brush-border enzymes; as a result, they are able to gain access to the lamina propria. Gluten-sequestering polymers and oral proteases may reduce the exposure of the immune system to immunogenic gluten peptides. Similar effects may be derived from zonulin antagonists or RhoA/ROCK inhibitors, all of which reduce epithelial permeability. Most gluten peptides that survive gastrointestinal breakdown are excellent substrates for TG2. The resulting deamidated products are recognised by CD4-positive T cells, when bound to HLA-DQ2 or HLA-DQ8 molecules on the cell surface of antigen-presenting cells. Therefore, TG2 inhibitors and HLA blockers are candidates for future coeliac disease therapy. Alternatively, activation of gluten-reactive T cells may be suppressed by peptide vaccines or by anti-CD3 treatment. Upon activation, gluten-reactive CD4-positive T cells produce IFN-γ, which is a major contributor to the development of the coeliac lesion. IFN-γ is also produced by intra-epithelial T cells. Therefore, anti-IFN-γ therapy could be considered a drug candidate. Similarly, IL-15, produced by either mononuclear cells in the lamina propria or by enterocytes themselves, attracts T cells with the capacity to kill enterocytes. IL-15 production is stimulated by gluten in the intestine in coeliac disease. Therefore, compounds that neutralise the effect of IL-15 are interesting drug candidates. Finally, B cells receive help from T cells to differentiate into plasma cells, which then produce autoantibodies against TG2. Because the interaction with CD20-positive B cells may amplify the anti-gluten T-cell response, anti-CD20 antibodies could be useful for the treatment of coeliac disease.
