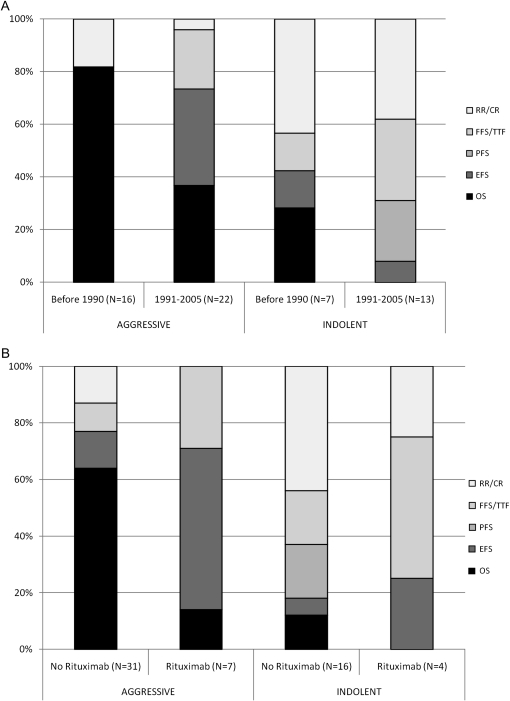
Figure 1.
Trends in selection of primary end points according to (A) time period and (B) presence or absence of rituximab in at least one treatment arm of the trial. RR/CR, response rate or complete response; FFS, failure-free survival/TTF, time-to-failure; PFS, progression-free survival; EFS, event-free survival; OS, overall survival.