Abstract
Recent studies have made significant progress toward the clinical implementation of high frequency conduction block (HFB) of peripheral nerves. However, these studies were performed in small nerves, and questions remain regarding the nature of HFB in large diameter nerves. This study in nonhuman primates shows reliable conduction block in large diameter nerves (up to 4.1 mm) with relatively low threshold current amplitude and only moderate nerve discharge prior to the onset of block.
Keywords: high frequency alternating current (HFAC), peripheral nerve, conduction block, primate, large diameter
Introduction
The delivery of high frequency alternating current (HFAC) to peripheral nerves can produce a reversible conduction block1. There is considerable hope that the method may be useful for the clinical treatment of disorders associated with pathological neural activity, such as spasticity or peripherally triggered pain. HFAC delivery has been the focus of several recent studies which have pursued an optimal waveform for delivery, optimal electrode design and a biophysical understanding of the mechanisms of this type of nerve conduction block. The results of these studies demonstrate that high frequency block (HFB) can be established quickly (≤ 1 sec to several seconds)2,3 and reversed quickly (≤ 1 sec)2. The induction of HFB requires the use of a frequency that is at least ~2 kHz4–7 and a waveform amplitude that is typically 3 V – 10 V (1 mA – 10 mA for current-controlled studies) across preparations4,6–9. The minimal waveform amplitude required to induce HFB, the block threshold, is dependent on the waveform frequency4,6,8,10,11 and geometry of the blocking electrode12. When HFAC is first applied to a nerve, it produces an intense volley of activity in the target nerve, the ‘onset response,’ before inducing block. The magnitude and duration of this onset response are also functions of the waveform frequency6,10, waveform amplitude1,6,10,13,14 and electrode geometry14. HFB has been successfully demonstrated in a chronic electrode preparation in the cat6 and likely results, mechanistically, from depolarization-induced inactivation of sodium channels15.
These recent studies have made significant progress toward the clinical implementation of HFB, particularly for myelinated fibers. However, these studies were performed in small nerves in the sea slug, frog, rat, and cat. It is not clear whether HFB can be achieved in large diameter nerves using similar waveform amplitudes and frequencies, and whether the onset response will be similar to that observed in small nerves. This study investigates the application of HFB to the large diameter median nerve in the monkey.
Methods
Experiments were performed in a total of three nerves in two adult male nonhuman primates (macaca fascicularis and macaca mulatta). Following induction of anesthesia with 10 mg/kg Ketamine, each monkey was intubated, and sedation was maintained with 2% isoflurane. Blood pressure, heart rate, respiratory rate and body temperature were monitored throughout the experiments and remained in a normal range. All animal care, surgical, and research procedures were consistent with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Northwestern University.
The left median nerve of the m. fascicularis (diameter 3.0 mm), and the left and right median nerves of the m. mulatta (diameters 3.9 and 4.1 mm, respectively) were evaluated. For each of the three nerve preparations, two 10–15 mm sections of nerve were dissected free of surrounding tissue, and two nerve cuff electrodes were placed around the exposed nerve. Both electrodes were made of silicone rubber with platinum contacts. The contacts were 9 mm by 1 mm rectangles with 2.5 mm intercontact spacing12. The proximal electrode was either bipolar or tripolar and was used to deliver 1.0 Hz supramaximal test stimuli (single monophasic pulses, 2.0 mA, 50µs – 300 µs). The distal electrode was bipolar and was used to deliver a 10 kHz, 20 kHz, 30 kHz or 40 kHz voltage-controlled (m. fascicularis) or current-controlled (m. mulatta) sinusoidal blocking waveform6,10. Each of the four fingers was tethered to a single series-loaded force transducer to monitor finger flexor force.
The block threshold and degree of onset response contraction (at threshold amplitude) were assessed at each frequency using previously established block-randomized protocols (three repeats/frequency/nerve for block thresholds and six repeats/frequency/nerve for onset response)10,12,14. The degree of onset response was quantified by integrating the flexor force over the first five seconds of approximately six-seconds of HFAC delivery (a period of six-seconds was used to minimize the effect of preparation fatigue on the onset response).
Results
HFAC delivery at frequencies of 20 kHz and higher resulted in a complete and reversible conduction block in each of the three median nerves tested. HFAC delivery at 10 kHz resulted in tetanic contraction in each nerve. 20 kHz stimulation resulted in a long duration (~30 sec) onset response for the 3.9 mm nerve. This frequency was not repeatedly evaluated for block threshold in this nerve to prevent muscle fatigue.
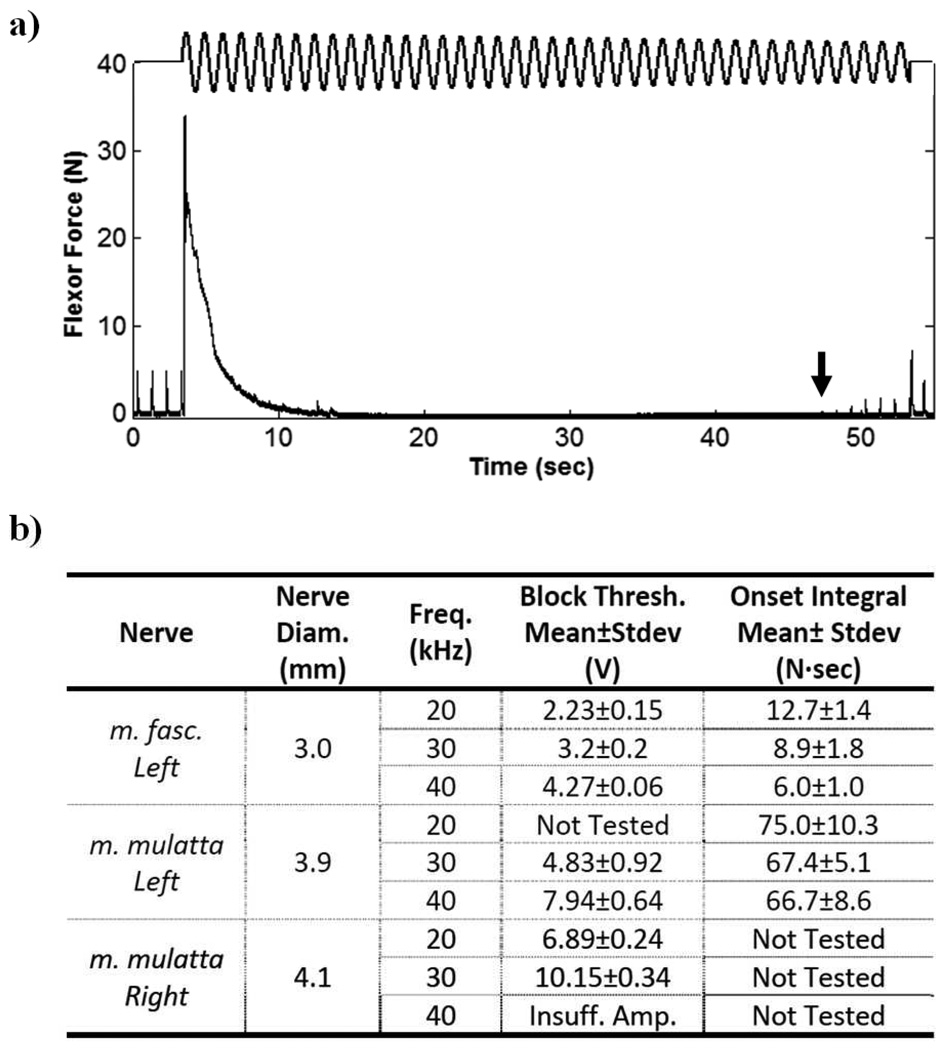
Figure 1a shows finger flexor force during a block threshold trial on the 3.9 mm nerve at a frequency of 30 kHz in which amplitude was decremented 0.1 V/sec to determine the minimum blocking amplitude. The initial onset response contraction lasted approximately 10 seconds. The absence of twitches during HFAC delivery indicated complete conduction block of the flexor motor axon pool. Figure 1b summarizes the results of the randomized block threshold and onset response trials for each of the three nerves. Block threshold values for the m. mulatta monkey have been converted to voltage values (although constant-current delivery was used) to allow for direct comparison with the m. fascicularis data. Values were converted using the measured blocking electrode impedance of ~500Ω for frequencies in the range tested. The table shows that block threshold correlated positively with nerve diameter. Larger nerves required higher amplitudes to induce HFB. Mean block thresholds increased with waveform frequency for each nerve tested, and the mean onset response integral showed a decreasing trend with frequency for each nerve. The effect of nerve diameter and frequency on block threshold were both found to be significant using ANOVA at the alpha = 0.05 level. The effect of nerve diameter on the integral of the onset response was also significant using ANOVA at the alpha = 0.05 level, however the effect of frequency was not significant. Muscle twitch force recovered to a pre-HFAC amplitude typically 30 – 120 seconds after cessation of blocking current delivery.
Figure 1.
a) Finger flexor force during HFAC block threshold trial on the left median nerve in the m. mulatta monkey at a frequency of 30 kHz. Relative HFAC amplitude is indicated diagrammatically. HFAC amplitude started at 15.0 V, and threshold was measured to be 10.6 V (the arrow indicates the first twitch resulting from partial conduction block). b) Table summarizing the results of the randomized block threshold and onset response trials for each of the three nerves tested. The waveform generator was unable to produce sufficient amplitude to produce a complete conduction block at 40 kHz for the 4.1 mm nerve.
Discussion
This study has demonstrated that robust and reversible HFB is possible in large diameter nerves. Block thresholds were in the range of 2V – 10V for a frequency range of 20 kHz to 40 kHz, which is slightly larger than the amplitude range reported for the 1mm diameter rat sciatic nerve in this frequency range10. Our findings showed a positive correlation between nerve diameter and block threshold for the three nerves tested, a trend which has been suggested in simulation studies12,15. Electrode geometry has previously been shown to substantially impact onset response14. The difference in magnitude of onset response shown between the 3.0 mm and 3.9 mm nerves may be explained by an incomplete circumferential coverage of the nerve in the experiment on the 3.9 mm nerve. The onset response in the three nerves was similar in its rate of decay to that observed in other species for cases in which electrode geometries were not optimized for minimizing onset firing14.
This study demonstrates HFB in nonhuman primates, and is an important step toward the clinical implementation of HFB. It shows that block can be reliably achieved in large diameter nerves (up to 4.1 mm) with moderate threshold amplitudes and moderate onset response firing. This study suggests that human trials of HFAC would likely result in the desired HFB. However, more work is required to produce an electrical blockade without significant onset response16,17. Elimination of the onset response will be important for some applications.
Acknowledgements
This work was supported by NINDS Grant No. NS053603, The Searle Foundation Chicago Community Trust and NIBIB Grant No. R01-EB-002091.
Abbreviations
- HFB
High Frequency Block
- HFAC
High Frequency Alternating Current
- ANOVA
Analysis of Variance
References
- 1.Rosenblueth A, Reboul J. The Blocking and Deblocking Effects of Alternating Currents on Nerve. Am J Physiol. 1939;125(2):251–264. [Google Scholar]
- 2.Kilgore K, Bhadra N. Nerve conduction block utilizing high-frequency alternating current. Medical and Biological Engineering and Computing. 2004;42(3):394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- 3.Foldes E, Ackermann D, Bhadra N, Kilgore K. Counted Cycles Method to Quantify the Onset Activity in High-Frequency Peripheral Nerve Block. Proceedings of International Conf of the IEEE EMBS; 2009. pp. 614–617. [DOI] [PubMed] [Google Scholar]
- 4.Bhadra N, Bhadra N, Kilgore K, Gustafson KJ. High frequency electrical conduction block of the pudendal nerve. Journal of Neural Engineering. 2006;3(2):180. doi: 10.1088/1741-2560/3/2/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattel M, Gerard R. The inhibitory effect of high-frequency stimulation and the excitation state of the nerve. The Journal of Physiology. 1935;83:407–415. doi: 10.1113/jphysiol.1935.sp003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaunt R, Prochazka A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabil Neural Repair. 2009;23:615. doi: 10.1177/1545968308328723. [DOI] [PubMed] [Google Scholar]
- 7.Tai C, Roppolo JR, de Groat WC. Block of external Urethral Sphincter Contraction by High Frequency Electrical Stimulation of Pudendal Nerve. The Journal of Urology. 2004;172(5, Part 1):2069–2072. doi: 10.1097/01.ju.0000140709.71932.f0. [DOI] [PubMed] [Google Scholar]
- 8.Ackermann D, Foldes E, Bhadra N, Kilgore K. Conduction block of peripheral nerve using high frequency alternating currents delivered through an intrafascicular electrode. Muscle and Nerve. 2010;41(1):117–119. doi: 10.1002/mus.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson RP, Andrews BJ. Localized electrical nerve blocking. IEEE Transactions on Biomedical Engineering. 2005;52(3):362. doi: 10.1109/TBME.2004.842790. [DOI] [PubMed] [Google Scholar]
- 10.Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle and Nerve. 2005;32(6):782. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- 11.Joseph L, Butera RJ. Unmyelinated Aplysia nerves exhinit a non monotonic blocking response to high frequency stimulation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2009;17(6):537–544. doi: 10.1109/TNSRE.2009.2029490. [DOI] [PubMed] [Google Scholar]
- 12.Ackermann D, Foldes E, Bhadra N, Kilgore K. Effect of Bipolar Cuff Electrode Design on Block Thresholds in High Frequency Electrical Neural Conduction Block. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2009;17(5):469–477. doi: 10.1109/TNSRE.2009.2034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowman B, McNeal D. Response of Single Alpha Motorneurons to High-Frequency Pulse Trains. Appl Neurophysiol. 1986;49:121–138. doi: 10.1159/000100137. [DOI] [PubMed] [Google Scholar]
- 14.Ackermann D, Bhadra N, Foldes E, Kilgore K. Effect of Nerve Cuff Electrode Geometry on Onset Response Firing in Conduction Block of Whole Nerve Using High Frequency Alternating Currents. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2010 doi: 10.1109/TNSRE.2010.2071882. In Press, online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhadra N, Lahowetz E, Foldes S, Kilgore K. Simulation of high-frequency sinusoidal electrical block of mammalian myelinated axons. J Comput Neurosci. 2007;22:313–326. doi: 10.1007/s10827-006-0015-5. [DOI] [PubMed] [Google Scholar]
- 16.Ackermann D, Foldes E, Bhadra N, Kilgore K. Nerve Block Using Combined Thermoelectric Cooling and High Frequency Alternating Current: Electrical Block Without Onset Response. J Neuroscience Methods. 2010;193(1):72–76. doi: 10.1016/j.jneumeth.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermann D, Foldes E, Bhadra N, Kilgore K. Peripheral Nerve Block Using Combined High Frequency Alternating and Direct Currents for Mitigation of Onset Firing. Medical and Biological Engineering and Computing. 2010 doi: 10.1007/s11517-010-0679-x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]