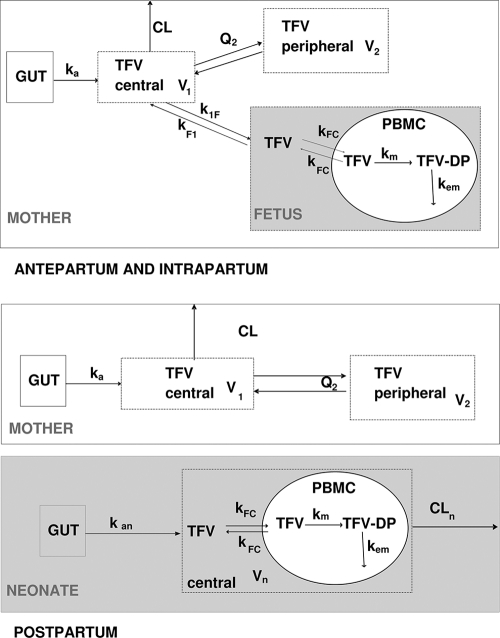
Fig. 1.
Population pharmacokinetic model for the simultaneous prediction of plasma tenofovir (TFV) concentrations in the mother, the cord, and the neonate and intracellular TFV and TFV-DP in the fetus and the neonate during intrapartum and postpartum. A two-compartment model with first-order absorption and elimination best described the maternal data. An “effect” compartment model linked to the maternal circulation best described cord concentrations. A one-compartment model with first-order absorption and elimination was sufficient to describe neonatal concentrations. An effect compartment linked to the fetal/neonatal circulation was used to describe TFV in PBMCs. An additional compartment was used for TFV-DP in PBMCs with first-order reactions for TFV to TFV-DP metabolism and TFV-DP elimination. F denotes the bioavailability, ka the maternal absorption rate constant, CL the maternal elimination clearance from the central compartment, V1 the volume of the central maternal compartment, Q2 the maternal intercompartmental clearance, V2 the volume of the peripheral maternal compartment, k1F the maternal-to-fetal rate constant, kF1 the fetal-to-maternal rate constant, kan the neonatal absorption rate constant, Vn/F the apparent neonatal volume of distribution, CLn/F the apparent neonatal elimination clearance; kFC the TFV cell transfer rate constant, km the TFV to TFV-DP metabolism constant rate, and kem the TFV-DP elimination constant rate.