Abstract
Adenosine suppresses immune responses through the A2A receptor (A2AR). This study investigated the interleukin 10 (IL-10) genetic profile and the expression of A2AR in peripheral blood mononuclear cells (PBMCs) of patients with mild cognitive impairment (MCI), Alzheimer disease (AD), and age-matched controls to verify, if they may help distinguish different forms of cognitive decline. We analyzed the IL-10 genotype and the expression of A2AR in 41 subjects with AD, 10 with amnestic MCI (a-MCI), 49 with multiple cognitive domain MCI (mcd-MCI), and 46 controls. There was a significant linear increase in A2AR mRNA levels and A2AR density from mcd-MCI to a-MCI, with intermediate levels being found in AD. The IL-10 AA genotype frequency was 67% in a-MCI, 46% in AD, 35% in mcd-MCI, and 20% in controls. These data suggest that the assessment of the IL-10 genotype and the expression of A2AR in PBMCs may be a valuable means of differentiating between a-MCI and mcd-MCI.
1. Introduction
The purine ribonucleoside adenosine (Ado) is a naturally occurring metabolite that is ubiquitously distributed throughout the body as a metabolic intermediary. Ado accumulates in the extracellular space at the site of inflammation [1] in response to metabolic stress and cell damage, and there is evidence that it could play a key role in preserving homeostasis [2]. The physiological responses to Ado take place as a result of the binding and activation of different transmembrane receptors: high-affinity A1 receptor (A1R) and A2AR, low-affinity A2BR, or low-abundance A3R. A2AR are found throughout the body, and an increasing wealth of evidence supports the idea that they represent the major immunoregulatory arm of the Ado receptor system, downregulating inflammation [3–7] and also preventing beta amyloid (Aβ) induced synaptotoxicity [8].
A recent study showed that stimulation of A2AR represents a crucial second signal in the production of IL-10, the major anti-inflammatory cytokine [9].
Inflammation is accepted to be a feature of Alzheimer's disease (AD) [10, 11] and the pathogenesis of neurodegeneration has been at least in part attributed to the release of proinflammatory cytokines from brain resident cells [12, 13] and, although less consistently, from peripheral cells [14, 15]. Recently, specific risk sets of pro-inflammatory and anti-inflammatory alleles were found to be associated with AD at different ages of onset [16]. Such alleles also comprise the −1082 polymorphism (G/A substitution) in the IL-10 gene promoter region [17]. On the basis of these considerations, we investigated (1) the expression of the A2AR gene and A2AR protein levels in the peripheral blood mononuclear cells (PBMCs) of patients with mild cognitive impairment (MCI, the preclinical state of dementia), patients with outright AD and age-matched controls without cognitive impairment and (2) the relationship between the IL-10 genetic profile and the A2A phenotype, since A2AR regulates IL-10 production.
2. Materials and Methods
2.1. Study Design
The study involved 41 AD patients (mean age 79.47 ± 6.30 years), 46 non demented age- and gender-matched healthy controls with similar educational levels (mean age 79.98 ± 6.36 years), and 59 subjects with MCI (mean age 78 ± 5.16 years). All of the patients were Caucasians living in Northern Italy who were prospectively enrolled from a larger population of outpatients attending the Geriatric Unit of the Ospedale Maggiore IRCCS, University of Milan, Italy.
Probable AD was diagnosed using standard clinical procedures and DMS IV and NINCDS-ADRDA criteria [18]. Functional status was assessed by means of the Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) scales and cognitive performance by means of the Mini-Mental State Examination (MMSE) and of an extensive neuropsychological evaluation. A computed tomography or magnetic resonance imaging scan corroborated the diagnosis of AD.
The criteria for the diagnosis of normal cognition were as follows: (1) no active neurological or psychiatric disorder; (2) no ongoing medical problems or related treatments interfering with cognitive function; (3) a normal neurological exam; (4) no psychoactive medications; (5) the ability to live and function independently in the community.
The MCI subjects were divided into two groups on the basis of their cognitive characteristics: amnestic MCI (a-MCI, 10 patients) and multiple cognitive domain MCI (mcd-MCI, 49 patients). The a-MCI subjects met the criteria described by Petersen [19]: only memory impairment (>1.5 SD above age- and education-specific norms) with no difficulties in any other area of cognitive functioning. The mcd-MCI subjects were impaired in at least two cognitive domains (>1 SD below the mean of an age- and education-matched population), their cognitive decline was self-reported or reported by a reliable informant, but they could not be diagnosed as having dementia. The cutoff score of 1 SD (less than the threshold used for a-MCI) was selected in order to ensure greater diagnostic sensitivity, albeit at the expense of diagnostic specificity. In fact the presence of more than one cognitive deficit and frequent initial IADL impairment may lead to mcd-MCI being mistaken for dementia, and the less restrictive criterion of >1 SD allows their better differentiation [20].
Blood from all patients and controls was collected in the morning between 8 and 9 AM, after a 6-hour fast.
None of the patients or controls was on treatment with dipyridamole or methylxanthines, and their caffeine consumption was about 80 mg/die (one cup of coffee) or less.
The risk of possible inflammatory processes was minimized by the fact that none of the subjects selected showed any clinical signs of inflammation (their body temperature was normal, and none of them had a concomitant inflammatory condition) and that they all had normal plasma albumin, transferring, and C-reactive protein levels. All of the subjects and their relatives gave their informed consent, and the study protocol was approved by the University Hospital's Ethics Committee.
2.2. Apolipoprotein E (ApoE) Genotyping
Whole blood was collected by means of a venipuncture into Vacutainers (Becton Dickinson Co., Rutherford, NJ). Genomic DNA was extracted using a previously described salting-out method [21], and its concentration and purity were determined by means of spectrophotometric analysis. The ApoE genotypes were determined by means of the PCR amplification of a 234 base-pair fragment of exon 4 of the ApoE gene, followed by digestion using Cfo1, as previously described [22]. Restriction patterns were revealed by means of 4% agarose gel electrophoresis.
2.3. Cytokine Genotyping
A polymerase chain reaction-sequence-specific primers (PCR-SSP) method was employed to assess IL-10 genotypes. The sequence in the promoter region of the IL-10 (polymorphism −1082) gene was amplified using the cytokine genotyping tray method (One Lambda, Canoga Park, CA, USA); the human β-globin gene was amplified as an internal control for the genomic DNA preparation. PCR conditions were indicated by the One Lambda PCR program (OLI-1), and the PCR products were visualized by electrophoresis in 2.5% agarose gel.
2.4. A2AR mRNA Expression
PBMCs were separated by density gradient using the Lympholyte-H kit (Cedarlane Laboratories Limited, Canada), and total RNA was isolated from 15 × 106 frozen cells using Chomczynski and Sacchi's modified method [23]. The concentration of total RNA was quantified by means of spectrophotometry, and 2 ug of RNA were reverse-transcribed using the M-MLV Reverse Transcriptase System and oligo (dT) (Clontech, Italy). The resulting cDNA was real-time amplified in a final volume of 50 μL. The mix contained 25 μL of 2x iQ SYBR Green Supermix (dNTPs, iTaq, and MgCl2; Bio-Rad, Italy) and 300 mM of each primer (A2AR: Forward 5′-GGCTGCCCCTACACATCATC-3′, Reverse 5′-GCCAGGTACATGAGCCAGAGA-3′). PCR was performed using a Chromo 4 instrument and analyzed using Opticon Monitor 2 (Celbio, Italy). All of the reactions were performed in duplicate, with thermal cycling conditions of 10 min at 95°C followed by 40 cycles at 95°C for 15 s, 56°C for 40 s, and 72C° for 30 s, with a ramp of 5C°/s. Real-time PCR for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed simultaneously (GAPDH: Forward 5′-ATTCCACCCATGGCAAATTC3′, Reverse 5′-TGGGATTTCCATTGATGACAAG-3′).
The relative quantification of A2A mRNA expression was carried out by using the comparative cycle threshold (Ct) method and the formula normalization ratio (NR) = 2-ΔΔCt. The ΔCt value of each sample was calculated as the Ct of the target gene minus the Ct of GAPDH, and then the ΔΔCt value was obtained as the difference between the ΔCt of the sample and the ΔCt of the calibrator [24]. According to this formula, the normalization ratio of the calibrator in each run is 1. The calibrator in each sample run was the same RNA extracted from a single healthy control and stored at −80°C.
2.5. A2A Receptor Densities
PBMCs from 10 a-MCI, 27 AD, 20 mcd-MCI, and 20 controls subjects were available for protein expression. The cells were lyzed in Triton X-114 Tris buffer with a protease inhibitors cocktail (Sigma, Italy). Briefly, after centrifugation (12,000 rpm at 4°C), the supernatant was loaded onto a sucrose cushion buffer and incubated at 37°C for 3 min. The samples were then centrifuged again in order to obtain two phases, and 10 μg of the total protein extracts of the aqueous phase were denatured by boiling for 10 min at 100°C in Laemmli SDS loading buffer, reduced with 5% mercaptoethanol and separated by 10% SDS polyacrylamide gel [25]. Electrophoresis was performed at 20–30 mA for 110 min, and the proteins were blotted onto a 0.45 μm polyvinylidene fluoride membrane (Immobilon, Millipore, Italy) at 90 V for 90 min at 4°C; after transfer, nonspecific binding was blocked for 120 min using 5% milk in phosphate buffer saline with 0.1% Tween 20 (PBST). The membranes were incubated overnight with polyclonal rabbit antihuman A2AR antibody (1 : 2000) (Calbiochem, Germany) and goat antirabbit IgG HRP (1 : 2000) (Bio-Sciences, Italy) and chemiluminescent substrate (Bio-Sciences, Italy). Purified A2AR (Chemicon, Italy) was loaded as positive control (one major band of about 45 kDa) and to evaluate the interassay variability (less than 10% in all experiments). To account for protein loading variation [26], the membranes were stripped and then reprobed with monoclonal antihuman GAPDH (1 : 8000) (Chemicon International, USA) and rabbit antimouse IgG-HRP (1 : 8000) (Sigma, Italy) and chemiluminescent substrate. All experiments were run in duplicate. Membranes were scanned, and the density of the protein bands was estimated by IM1D software (Bio-Sciences, Italy). The results were expressed as Arbitrary Units (A2AR /GAPDH ratio).
2.6. Statistical Analysis
The statistical analysis was performed using the SPSS statistical package (SPSS version 17, Chicago, IL). An outlier analysis was conducted before the comparisons. The differences in mRNA and protein levels, expressed as mean values ± standard error (SE), were calculated using the one-way analysis of variance followed by Bonferroni's post hoc test in the case of multiple comparisons. The genotype and allele distributions were evaluated using Pearson's χ2. A P value <.05 was considered statistically significant.
3. Results
3.1. Distribution of the ApoE Genotype
The frequency of ApoE ε4 was 50% in a-MCI patients, 44% in AD patients, 38.8% in mcd-MCI patients, and 11% in control subjects. These frequencies are similar to those already reported [27].
3.2. Distribution of the IL-10 Genotype
The distribution of the −1082 (G/A) polymorphism was similar to that previously described [22]. In particular, the frequency of the AA genotype, associated with a higher risk of AD, was 67% in a-MCI, 46% in AD, 35% in mcd-MCI, and 20% in controls, respectively (P = .014).
3.3. A2A Gene Expression and Receptor Densities
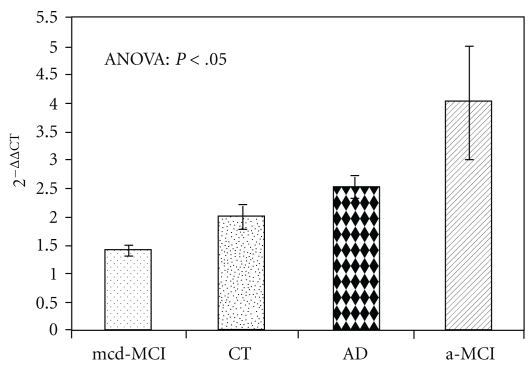
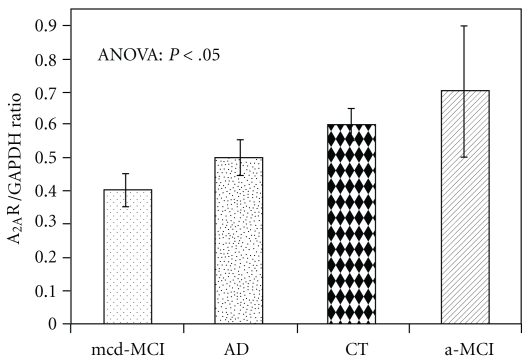
The qPCR analysis (Figure 1) revealed significantly higher (P < .05) A2AR mRNA levels in patients with a-MCI (3.77 ± 1.02) than in controls (2.12 ± 0.21) and in patients with mcd-MCI (1.42 ± 0.12). It is interesting to note that also A2AR density (Figures 2 and 3), expressed as A2AR/GAPDH ratio, was significantly higher in patients with a-MCI (0.71 ± 0.17) than in controls (0.62 ± 0.05) and in patients with mcd-MCI (0.43 ± 0.05). Both gene expression (2.50 ± 0.22) and density (0.50 ± 0.06) of the A2AR in PBMCs of AD patients were not significantly different from those of the controls (Figures 1 and 3). When the data were stratified according to the presence or absence of the ApoE ε4 allele, A2AR gene expression and receptor density were similar both in the ε4 carriers and noncarriers.
Figure 1.
A2AR mRNA levels (mean values ± standard error) in PBMCs from 41 AD, 10 a-MCI, 49 mcd-MCI, and 46 control (CT) subjects quantified by the 2-ΔΔCt method. Bonferroni post hoc: a-MCI versus controls and mcd-MCI patients (P < .05).
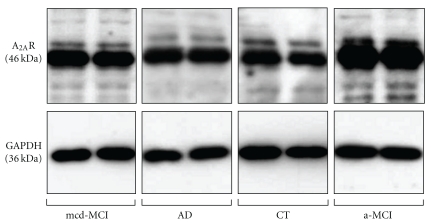
Figure 2.
Paradigmatic example of the Western blot analysis of the A2AR densities in PBMC extract from one subject belonging to the AD, a-MCI, mcd-MCI, or control (CT) group, run in duplicate.
Figure 3.
A2AR densities (mean values ± standard error) in PBMCs from 27 AD, 10 a-MCI, 20 mcd-MCI, and 20 control (CT) subjects expressed as A2AR/GAPDH ratio. Bonferroni post hoc: a-MCI versus controls and mcd-MCI patients (P < .05).
Interestingly, the carriers of the G allele of the −1082 IL-10 polymorphism (GG and GA) showed the lowest level of A2AR mRNA, independently of cognitive status (1.7 ± 0.13 and 2.7 ± 0.36, in carriers versus noncarriers, resp.; P = .002).
4. Discussion
The results of this observational study show that the level of A2AR expression is higher in PBMCs from a-MCI than from mcd-MCI. When the data were stratified according to the presence or absence of the ApoE ε4 allele, A2AR gene expression and receptor density were similar both in the ε4 carriers and noncarriers. This indicates that the ApoE ε4 allele, a major genetic risk factor for late onset sporadic AD [28–30], does not appear to participate in the modulation of this gene.
The small number of patients and the lack of A2AR measurements in the cerebrospinal fluid do not allow us to draw any conclusion on the relationship between peripheral A2AR modulation and Ado metabolism in the brain. Yet the finding of an upregulation of A2AR in the PBMCs of subjects with a-MCI but not with AD seems to fit with the results of a previous study [31] demonstrating that in the brain cortex the increased expression of both A2AR and A1R is mainly an early event in the pathogenesis of AD [32]. One possible explanation of our finding is that a second event, which is required for the development of AD in a-MCI subjects, subsequently intervenes to downregulate A2AR expression in PBMCs by a negative feedback mechanism and thus counteracts the anti-inflammatory effect of Ado. Therefore, within the multifactorial pathogenesis of AD, there may be a specific time-window for the involvement of the Ado system.
It is still unclear whether and how peripheral inflammation acts on the AD brain; there are, however, several lines of evidence in favour of its contribution to the disease process. First, AD patients who also have short-term peripheral infections undergo a sudden decline in cognitive status [33]; second, plasma levels of alpha1-antichymotrypsin, interleukin-6, and C-reactive protein are increased before the clinical onset of various types of dementia, including AD [34]; third, the signs of inflammation observed in the brain of AD patients are comparable to those seen in peripheral inflammatory reactions and are likely to have a strong cytotoxic effect on neurons [35]; fourth, individuals who have levels of Aβ and tau aggregates similar to those of AD patients, but yet do not develop dementia, exhibit lesser signs of inflammation [36].
The lack of a significant difference in A2AR expression between AD subjects and controls is consistent with the data of former studies showing that also cytokine production is increased in PBMCs of MCI but not AD subjects [15, 37]. Thus, a peripheral inflammatory response seems to be involved only in the early stage of the disease and appears to be lost in overt AD.
In our patients the IL-10 low-producer AA genotype, which also seemed to be associated with the highest mRNA levels of A2AR, was more frequent in the a-MCI group. IL-10, a powerful anti-inflammatory cytokine, maps to chromosome 1 between 1q31 and 1q32, is highly polymorphic, and its production is correlated to biallelic polymorphisms at position−1082 (G to A). In previous studies, we not only described a significantly greater prevalence of the IL-10 −1082 AA low-producer genotype in subjects with overt AD as well as in subjects with a-MCI who eventually progressed to AD [37], but we also found a reduced IL-10 generation in PBMCs from these patients after Aβ stimulation [22]. Interestingly a report on centenarians, in whom an exceptionally long lifespan is held to reflect the combined influence of lifestyle choices and genetic factors, demonstrated that longevity is significantly associated with the IL-10 high-producer GG genotype [38].
Limitations of this observational study are the small number of enrolled subjects, and that it was possible to evaluate the correlation between the IL-10 genetic profile and gene expression only for A2AR. However, if the findings of this study are confirmed by a prospective study with a larger number of subjects, they can have a potential clinical application and provide further insight into the pathogenesis of AD. The evaluation of A2AR expression in the periphery could be a valuable means to differentiate mcd-MCI and a-MCI subjects. Also, it could be hypothesized that the overall risk of developing AD is governed by a multifactorial “susceptibility profile” related not only to gene variants but also to alterations in the signalling pathway of molecules, such as adenosine, that play a key role in neuroinflammation.
References
- 1.Haskó G, Sitkovsky MV, Szabó C. Immunomodulatory and neuroprotective effects of inosine. Trends in Pharmacological Sciences. 2004;25(3):152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature Reviews Drug Discovery. 2008;7(9):759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological Reviews. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 4.Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2000;362(4-5):364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- 5.Erdmann AA, Gao ZG, Jung U, et al. Activation of Th1 and Tc1 cell adenosine A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105(12):4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lappas CM, Rieger JM, Linden J. A adenosine receptor induction inhibits IFN-γ production in murine CD4 T cells. Journal of Immunology. 2005;174(2):1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 7.Lappas CM, Sullivan GW, Linden J. Adenosine AA agonists in development for the treatment of inflammation. Expert Opinion on Investigational Drugs. 2005;14(7):797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- 8.Canas PM, Porciúncula LO, Cunha GMA, et al. Adenosine A receptor blockade prevents synaptotoxicity and memory dysfunction caused by β-amyloid peptides via p38 mitogen-activated protein kinase pathway. Journal of Neuroscience. 2009;29(47):14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csóka B, Haskó G. Adenosine, inflammation pathways and therapeutic challenges. Joint Bone Spine. 2011;78(1):4–6. doi: 10.1016/j.jbspin.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nature Medicine. 2006;12(9):1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 11.Querfurth HW, LaFerla FM. Alzheimer’s disease. The New England Journal of Medicine. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 12.Tarkowski E, Blennow K, Wallin A, Tarkowski A. Intracerebral production of tumor necrosis factor-α, a local neuroprotective agent, in Alzheimer disease and vascular dementia. Journal of Clinical Immunology. 1999;19(4):223–230. doi: 10.1023/a:1020568013953. [DOI] [PubMed] [Google Scholar]
- 13.Tarkowski E, Liljeroth AM, Nilsson A, Minthon L, Blennow K. Decreased levels of intrathecal interleukin 1 receptor antagonist in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2001;12(5):314–317. doi: 10.1159/000051276. [DOI] [PubMed] [Google Scholar]
- 14.Bermejo P, Martín-Aragón S, Benedí J, et al. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer’s disease. Immunology Letters. 2008;117(2):198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S. Systemic immune aberrations in Alzheimer’s disease patients. Journal of Neuroimmunology. 2008;193(1-2):183–187. doi: 10.1016/j.jneuroim.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Licastro F, Porcellini E, Caruso C, Lio D, Corder EH. Genetic risk profiles for Alzheimer’s disease: integration of APOE genotype and variants that up-regulate inflammation. Neurobiology of Aging. 2007;28(11):1637–1643. doi: 10.1016/j.neurobiolaging.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Lio D, Licastro F, Scola L, et al. Interleukin-10 promoter polymorphism in sporadic Alzheimer’s disease. Genes and Immunity. 2003;4(3):234–238. doi: 10.1038/sj.gene.6363964. [DOI] [PubMed] [Google Scholar]
- 18.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.Zanetti M, Ballabio C, Abbate C, Cutaia C, Vergani C, Bergamaschini L. Mild cognitive impairment subtypes and vascular dementia in community-dwelling elderly people: a 3-year follow-up study. Journal of the American Geriatrics Society. 2006;54(4):580–586. doi: 10.1111/j.1532-5415.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16(3):p. 1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arosio B, Trabattoni D, Galimberti L, et al. Interleukin-10 and interleukin-6 gene polymorphisms as risk factors for Alzheimer’s disease. Neurobiology of Aging. 2004;25(8):1009–1015. doi: 10.1016/j.neurobiolaging.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nature Protocols. 2006;1(2):581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2T method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Dittmer A, Dittmer J. β-Actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27(14):2844–2845. doi: 10.1002/elps.200500785. [DOI] [PubMed] [Google Scholar]
- 27.Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Annals of Human Biology. 2006;33(3):279–308. doi: 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- 28.Berlau DJ, Corrada MM, Head E, Kawas CH. ApoE ε2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72(9):829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bu G. Apolipoprotein e and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nature Reviews Neuroscience. 2009;10(5):333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Basak JM, Holtzman DM. The Role of Apolipoprotein E in Alzheimer’s Disease. Neuron. 2009;63(3):287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albasanz JL, Perez S, Barrachina M, Ferrer I, Martín M. Up-regulation of adenosine receptors in the frontal cortex in Alzheimer’s disease. Brain Pathology. 2008;18(2):211–219. doi: 10.1111/j.1750-3639.2007.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature Reviews Immunology. 2007;7(2):161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 33.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of Neurology. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 34.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lue LF, Brachova L, Civin WH, Rogers J. Inflammation, Aβ deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. Journal of Neuropathology and Experimental Neurology. 1996;55(10):1083–1088. [PubMed] [Google Scholar]
- 36.Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Experimental Gerontology. 2007;42(3):233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arosio B, Mastronardi L, Vergani C, Annoni G. Intereleukin-10 promoter polymorphism in mild cognitive impairment and in its clinical evolution. International Journal of Alzheimer's Disease. 2010;2010:5 pages. doi: 10.4061/2010/854527. Article ID 854527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lio D, Scola L, Crivello A, et al. Gender-specific association between—1082 IL-10 promoter polymorphism and longevity. Genes and Immunity. 2002;3(1):30–33. doi: 10.1038/sj.gene.6363827. [DOI] [PubMed] [Google Scholar]