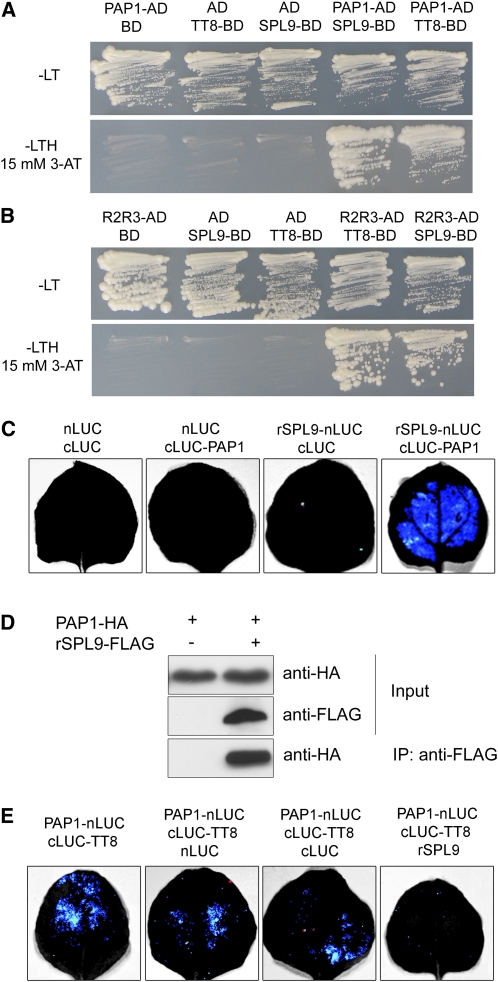
Figure 6.
SPL9 Binds to PAP1.
(A) SPL9 and TT8 binding to PAP1 in yeast. TT8 and SPL9 were in-frame fused to the GAL4 binding domain (BD) in pGBKT7, whereas PAP1 was fused to the GAL4 activation domain (AD) in pGADT7. Transformed yeast cells were grown on SD –Leu Trp (–LT) (top). The direct interactions between TT8-BD, SPL9-BD, and PAP1-AD were assayed on a SD –Leu Trp His (–LTH) plate, supplemented with 15 mM 3-amino-1,2,4,-triazole (3-AT) (bottom). The pGADT7 (AD) and pGBKT7 (BD) were used as controls.
(B) Role of R2R3 domain of PAP1 in mediating binding of PAP1 to SPL9 in yeast. The R2R3 domain of PAP1 was fused to AD in pGADT7.
(C) BiFC assay. The leaves of N. benthamiana were infiltrated with agrobacteria as indicated. Blue luminescence indicates a direct protein–protein interaction between rSPL9 and PAP1. Constructs were combined at a 1:1 ratio.
(D) CoIP analysis. Proteins were transiently expressed in N. benthamiana. Protein was immunoprecipitated with anti-FLAG antibody, and the IP fraction was analyzed in a protein blot with anti-HA antibody. Input fraction was analyzed by immunoblotting using either anti-FLAG or anti-HA antibody.
(E) Competition of SPL9 and TT8 for PAP1 binding. Constructs were combined at a 1:1:4 ratio for PAP1-LUC-N: LUC-C-TT8: LUC-N, LUC-C, or rSPL9. Blue luminescence indicates a direct interaction between TT8 and PAP1.