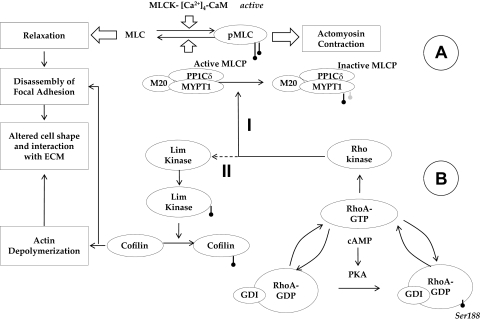
Figure 12.
Crosstalk between cAMP-PKA and RhoA-Rho kinase in the signaling of actomyosin contraction. (A) Overview of MLC phosphorylation: MLC kinase (MLCK) drives phosphorylation and MLC phosphatase (MLCP) induces the dephosphorylation of MLC. MLCP is a trimeric complex consisting of a catalytic subunit (PP1Cδ), a myosin-binding subunit (MYPT1), and a subunit of unknown function (M20). Phosphorylation of MYPT1 by Rho kinase at Thr853 inactivates PP1Cδ, leading to sustained actomyosin contraction. (B) Impact of elevated cAMP on actin cytoskeleton: Protein kinase A (PKA), an effector of cAMP, phosphorylates RhoA at Ser188, which increases the affinity of the small GTPase to its GDI. Thus, PKA blocks RhoA activation and thereby reduces the phosphorylation of MYPT1 (Mechanism I) and Lim kinase (Mechanism II). Through Mechanism I, actomyosin contraction is directly reduced. On the other hand, Mechanism II results in the activation of cofilin (by dephosphorylation), causing depolymerization of the actin cytoskeleton, which results in a reduction in stress fibers. In summary, through the inhibition of RhoA, cAMP induces the loss of actomyosin contraction. This loss, in turn, weakens the focal adhesions, which manifests as a change in cell morphology.