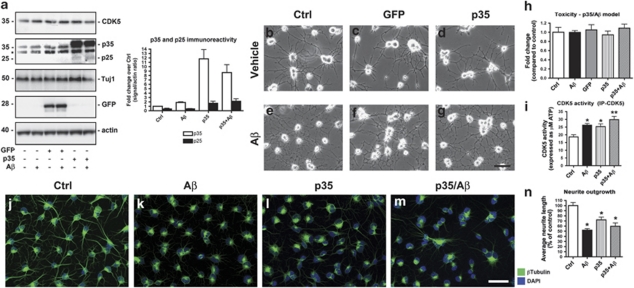
Figure 1.
Characterization of an in vitro model of adult neurogenesis in AD, and impaired neurite outgrowth with aberrant CDK5 activation. Differentiating NPCs were infected at day 2 with an adenoviral vector expressing p35 or GFP as a control, and 24 h later, cells were exposed to 1 μM Aβ1−42. Cells were analyzed by live cell microscopy, or lysed for immunoblot analysis, or briefly extracted, and then fixed with glutaraldehyde and processed for β-tubulin immunofluorescence. (a) Immunoblot analysis of the expression levels of CDK5, p35/p25, β-III-tubulin (Tuj1), GFP, and actin (loading control) in NPC-derived neural progeny expressing adenovirus-delivered p35 or GFP (vector control) and treated with Aβ1−42 for 24 h. Graph showing quantitative analysis of p35/p25 immunoreactivity demonstrates increased p25 generation in p35-expressing cells after Aβ treatment. *Nonspecific immunoreactive band detected with the p35/p25 antibody. (b–g) Live cell imaging showing the morphology of NPC-derived neural progeny expressing p35 or GFP control and treated with Aβ. (h) No significant cytotoxicity (measured by LDH assay) was detected in cells infected with p35 adenovirus or GFP control, or with Aβ treatment alone or in combination with p35 expression. (i) Increased kinase activity of CDK5 in p35 and Aβ alone and p35/Aβ-treated NPC-derived neural progeny. (j–m) Compared with vehicle-treated controls (Ctrl), shorter β-tubulin-immunoreactive neurites were detected in NPC-derived neural progeny with p35 or Aβ treatment alone or in combination. Cell nuclei were co-stained with DAPI reagent. (n) Quantitative image analysis showing reduced neurite lengths in NPC-derived neural progeny with p35 or Aβ treatment alone or in combination. Scale bar=10 μm. *P<0.05 compared with vehicle-treated controls by one-way ANOVA with post hoc Dunnett's test (N=3). **P<0.01 compared with vehicle-treated controls by one-way ANOVA with post hoc Dunnett's test (N=3)