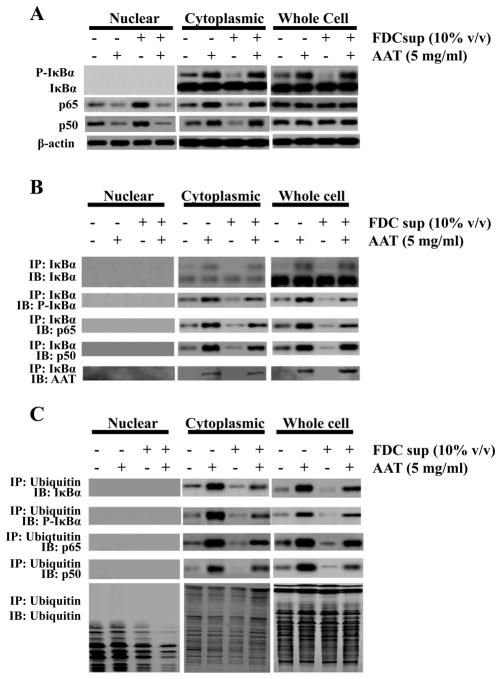
Figure 2.
AAT blocks NF-κB activation despite phosphorylation and ubiquitination of IκBα. (A) Peripheral blood CD4+ T cells were isolated, stimulated and infected following the procedure in Materials and Methods. After 24 h of incubation with or without FDC supernatant (10% v/v) and in the presence or absence of AAT (5 mg/ml), the nuclear, cytoplasmic and whole cell proteins were extracted and subjected to immunoblotting for NF-κB components p50, p65 and the non- and phosphorylated forms of the NF-κB inhibitor, I Ba. As expected, β-actin was detected in both the cytoplasmic and the nuclear fractions from the CD4+ T cells (33–35). (B) Cells treated as in A were subjected to immunoprecipitation using I Ba-specific Ab and the precipitated complexes probed using the indicated Abs for IκBα, phosphorylated IκBα, and the NF-κB components p65 and p50. (C) Cells treated as in A were subjected to immunoprecipitation with ubiquitin-specific Ab and the isolated complexes subjected to immunoblotting for IκBα, phosphorylated IκBα, NF-κB p65 and p50 components and ubiquitin as indicated. The data presented in these panels are representative of 3 independent experiments.