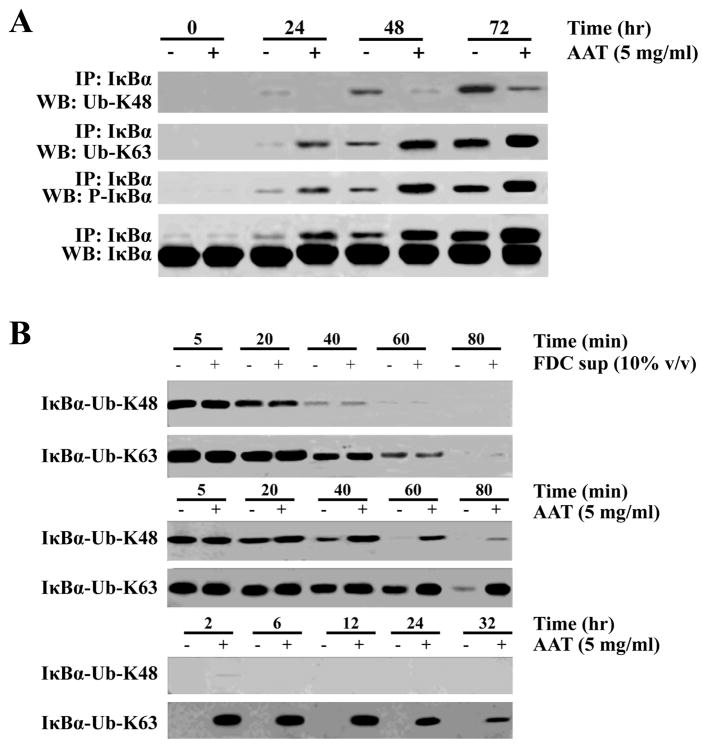
Figure 6.
AAT treatment alters polyubiquitination linkages resulting in the prolonged half-life of IκBα in CD4+ T cells. (A) CD4+ T cells were treated as before and incubated in the presence or absence of AAT (5 mg/ml) and harvested at the indicated times, lysed and IκBα was immunoprecipitated and subjected to immunoblotting with Abs specific for polyubiquitination on either ubiquitin lysine residue K48 or K63. (B) Cells were treated as in Figure 5 to radiolabel proteins (pulse-chase) and after immunoprecipitation of IκBα at the indicated times, the agarose A/G beads were boiled and the supernatant collected. The IκBα complexes were subjected to a second immunoprecipitation using Abs specific for polyubiquitination linked through ubiquitin residues K48 or K63. The precipitated complexes were then examined using a phosphorimager to detect the radiolabeled IκBα that remained over the period of culture. The top two rows of this panel present data from CD4+ T cell cultures with or without FDC supernatant while the remaining rows are from cells cultured with or without AAT but not FDC supernatant.