Fig. 2.
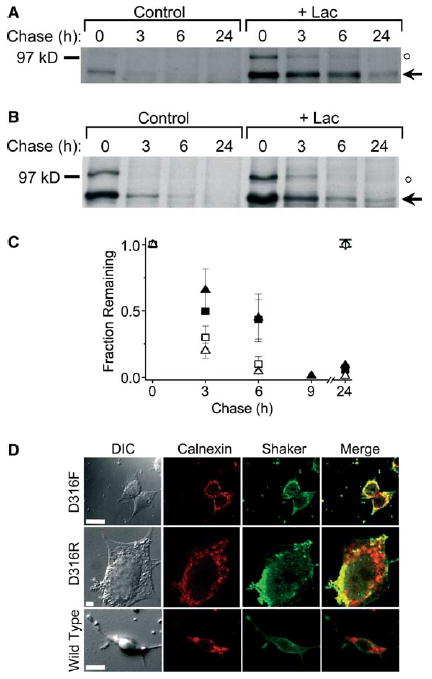
Different D316 mutations target the Shaker protein to proteasomes for degradation. (A,B) D316F and D316R mutant Shaker proteins were metabolically labeled and treated as described under Fig. 1. Representative fluorographs are shown for D316F (A) and D316R (B). The arrow indicates the position of the immature, core-glycosylated mutant protein. An unstable band of unknown identity with an apparent molecular weight of 100 kDa is also visible (denoted by ○, see Fig. 1 legend). (C) Summary of densitometric analysis of turnover for D316F (open squares, n = 3), D316F + Lac (filled squares, n = 3), D316R (open triangles, n = 3; except n = 1 for the 9 h and n = 2 for the 24 h time points) and D316R + Lac (filled triangles, n = 3; except n = 1 for the 9 h and n = 2 for the 24 h time points). The amount of protein in the bands was quantified by densitometry, normalized to the amount of immature Shaker protein at time 0, and plotted versus chase time. The t1/2 for the D316F mutant was estimated to be 1 h in the absence and 5 h in the presence of Lac. The t1/2 for the D316R mutant was estimated to be 1.5 h in the absence and 3 h in the presence of Lac. The diamond symbols have the same meaning as in Fig. 1B and D. Data are provided as mean ± S.E.M. except for the 9 and 24 h time points. (D) Representative differential image contrast and confocal images of HEK293T cells transiently transfected with D316F, D316R, or wild-type Shaker. Forty eight hours post-transfection, cells were permeabilized and labeled with a rabbit polyclonal antiserum directed against a Shaker-β-galactosidase fusion protein and a mouse monoclonal antibody directed against calnexin (an ER-resident protein) and visualized by incubation with fluorescent-conjugated Alexa-488 goat anti-rabbit (Shaker, green) and Alexa-568 goat anti-mouse (calnexin, red) secondary antibodies, respectively. For each panel, the same confocal plane was used for acquisition. Yellow regions in the merged images represent co-localization of mutant Shaker proteins and calnexin. Calibration bar, 10 μm.
