Fig. 3.
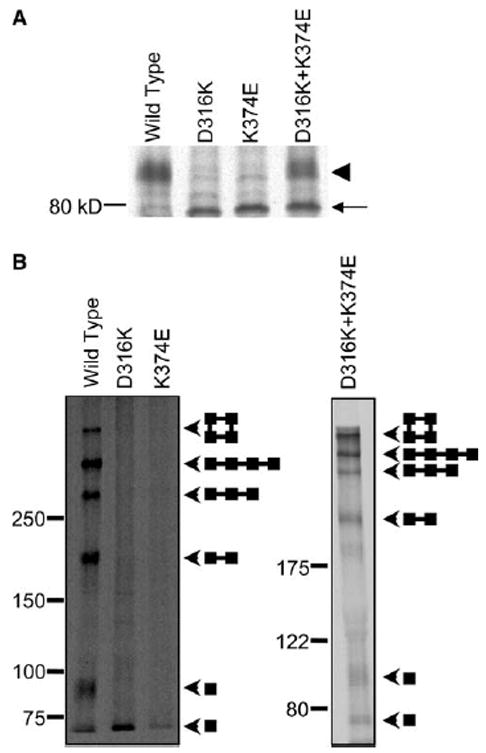
D316K + K374E protein forms C96/C505 disulfide bond in mammalian cells. (A) The wild-type, D316K, K374E, and D316K + K374E proteins were expressed in HEK293T cells, metabolically labeled for 30 min and chased for 3 h in non-radioactive medium. Shaker protein was immunoprecipitated, and electrophoresis was performed under reducing conditions. A representative fluorograph is shown (n = 3). The arrowhead indicates the position of the mature, complex glycosylated protein. The arrow indicates the position of the immature, core-glycosylated protein. (B) Intact cells were metabolically labeled for 30 min, chased in non-radioactive medium for 3 h, and oxidized with 100 mM hydrogen peroxide for 15 min. Remaining free sulfhydryl groups were protected with N-ethylmaleimide. Shaker protein was immunoprecipitated, and electrophoresis was performed under non-reducing conditions on 5–20% gradient (left panel) or 7.5% (right panel) SDS–PAGE gels. Arrowheads indicate the positions of the bands corresponding to monomer, dimer, trimer, linear tetramer, and circular tetramer. Diagrams show schematically the predicted structures of each band [3].
