Fig. 4.
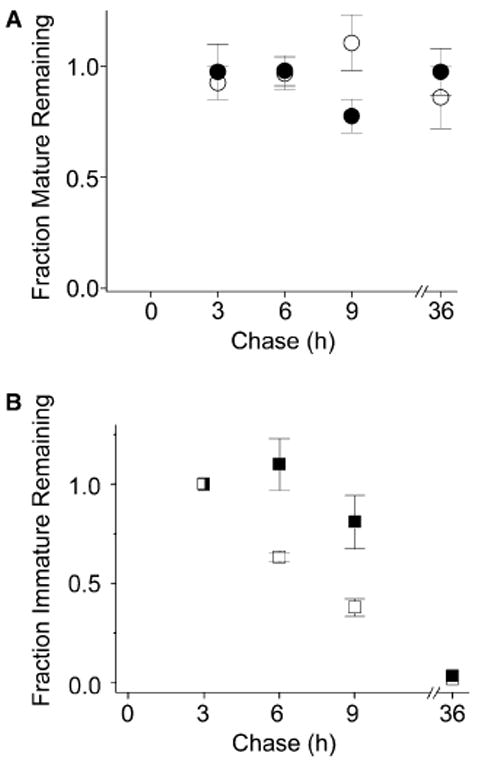
The mature and immature forms of the D316K + K374E protein are differentially stable. The D316K + K374E double mutant was metabolically labeled and treated as described under Fig. 1. (A) Summary of densitometric analysis of protein turnover for the mature band of D316K + K374E (open circles, n = 3) and D316K + K374E + Lac (filled circles, n = 3). The amount of protein in the mature band was quantified by densitometry, normalized to the amount of mature Shaker protein present at time 3 h, and plotted versus chase time. (B) Summary of densitometric analysis of protein turnover for the immature band of D316K + K374E (open squares, n = 3) and D316K + K374E + Lac (filled squares, n = 3). The amount of protein in the immature band was quantified by densitometry, normalized to the amount of immature Shaker protein present at time 3 h, and plotted versus chase time. Note that at time 0, the total D316K + K374E protein is in the form of an immature band that represents a mixed population. About 50% of this protein will mature, a process that should be complete by approximately 2 h of chase [27]. After 3 h of chase, maturation is expected to be complete, so further change in the amounts of the mature and immature bands primarily reflects turnover. Therefore, the turnover of the mature and immature bands was assessed relative to the 3 h time point. The half time of degradation (t1/2) for the immature form of D316K + K374E was estimated to be 4.5 h in the absence of Lac, a value that is intermediate between estimated t1/2 values for the D316K and K374E single mutants, which were 2 and 15 h, respectively (see Fig. 1 legend).
