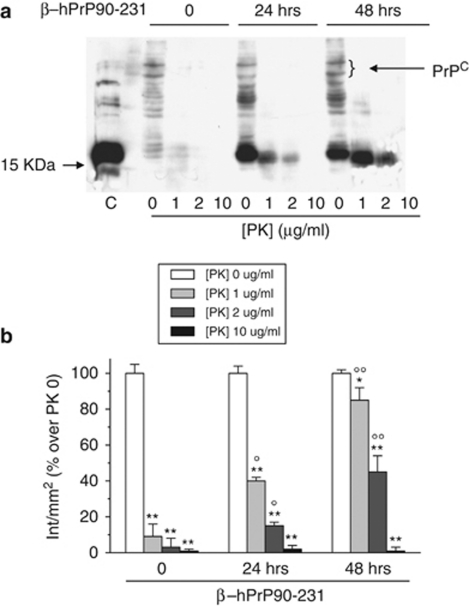
Figure 5.
Internalized β–hPrP90-231 is partly PK resistant. (a) Cells treated with PBS or β–hPrP90-231 (1 μM) for 24 and 48 h were collected in non-denaturing lysis buffer, without protease inhibitors. Proteins for each sample (100 μg) were digested with increasing concentrations of PK (1, 2, and 10 μg/ml) at 37°C for 30 min. PrPC and β–hPrP90-231 digestion fragments were resolved by 15% SDS-PAGE using the anti-PrP antibody 3F4. β–hPrP90-231 (100 ng) was loaded to compare the electrophoretic run of PrP fragment before and after intracellular uptake (lane C). Control cells evidence a faint 3F4-immunoreactive signal between 35 and 45 kDa, corresponding to PrPC, that is almost fully digested by PK. Cell treatment with β–hPrP90-231 for 24 and 48 h induced the appearance of a 16 kDa band corresponding to internalized PrP fragment that showed a full digestion only at 10 μg/ml PK, indicating that internalized β–hPrP90-231 is significantly more protease-resistant than PrPC. (b) Densitometric analysis of 3F4 signal in untreated (white columns) and PK-digested samples (light gray, gray, and black columns). Time 0, in the absence of β–hPrP90-231, indicates PK effects on PrPC (MW 35–45 kDa). Plot shows that intracellular β–hPrP90-231 resistance is higher than that of PrPc and increases with treatment time. Values (n=3) represent OD intensity/mm2 and expressed as percentage of respective PK-undigested samples. *P<0.05 and **P<0.01 versus respective undigested controls; OP<0.05, OOP<0.01 versus corresponding PK digestion of vehicle-treated cells