Abstract
Background
Baseline characteristics from the adult cohort of a randomized controlled trial comparing sensor-augmented pump (SAP) and multiple daily injection (MDI) therapy were analyzed for significant relationships with −0.5% A1C change at 1 year of therapy without incidence of severe hypoglycemia (defined as A1C benefit).
Methods
Baseline characteristics were compared with A1C benefit. Statistically significant predictors were analyzed further to determine appropriate cutpoints of relative A1C benefit.
Results
Baseline A1C ≥9.1%, age at randomization ≥36 years, and age at diabetes diagnosis of ≥17 years were associated with a greater SAP benefit relative to MDI than other cutpoints.
Conclusions
People with type 1 diabetes who had a high A1C and who were older at diagnosis and older at randomization experienced the most benefit from SAP therapy.
Introduction
Among adult subjects in the Sensor-Augmented Pump Therapy for A1C Reduction (STAR) 3 study,1,2 which compared sensor-augmented pump (SAP) to multiple daily injection (MDI) therapy for 1 year, SAP therapy resulted in a greater 1-year absolute change in A1C compared with continued MDI therapy.1 (Participating centers are listed in the Appendix.) Initiating SAP therapy is expensive and requires a substantial time commitment on the part of both patient and provider. Therefore, it may be useful to identify baseline factors in adults that are associated with an enhanced or diminished benefit of SAP relative to continuing MDI.
Presentations that reported portions of this research have been published in abstract form.3,4
Subjects and Methods
Study conduct
Study eligibility criteria included type 1 diabetes, 7–70 years of age, use of MDI with a long-acting insulin analog in the previous 3 months, A1C between 7.4 and 9.5%, and two or fewer severe hypoglycemic events (defined as ones absolutely requiring assistance from another person and preferably accompanied by a confirmatory blood glucose measurement of <50 mg/dL) in the previous year. Subjects were randomized to SAP therapy (MiniMed Paradigm® REAL-Time System, Medtronic Inc., Northridge, CA) with insulin aspart or MDI using insulin aspart and insulin glargine. Clinicians and subjects optimized therapy on an individual basis, and A1C was obtained at quarterly visits. Training and visit schedules were similar between groups. All subjects completed 1 week of continuous glucose monitoring (CGM) at baseline, 6 months, and 1 year to collect glycemic outcomes from both study groups.1,2 Baseline and in-study characteristics of pediatric subjects as predictors of A1C benefit will be presented elsewhere.
Statistical methods
Baseline characteristics were analyzed to determine if they predicted ≥0.5% A1C reduction without severe hypoglycemia at 1 year using a univariate logistic regression model and a multivariate logistic regression model that used a backward elimination procedure to keep variables with P < 0.05. Receiver operating characteristic (ROC) curves determined whether key baseline characteristics predicted a ≥0.5% reduction in A1C without severe hypoglycemia at 1 year and were adjusted for baseline A1C and mean sensor glucose value. C-scores (equivalent to areas under the curve) of different categories were compared using a nonparametric test. Treatment differences for subjects with certain baseline characteristics were derived from an analysis of covariance model that included categorical variables (treatment group and age at randomization, age at diagnosis, or baseline A1C) and continuous variables (mean glucose and baseline A1C) as fixed effects.
Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). All P values are two-sided.
Results
Subjects
There were 495 subjects randomized in STAR 3, including 336 adults (169 to SAP, 167 to MDI). Seven adults did not have a follow-up A1C. Of the resulting intention-to-treat analysis population of 329 adults, 24 withdrew from the study before completing the 1-year follow-up. Adults were similar between groups at baseline in all characteristics except mean weight and student status, which were significantly higher among MDI subjects (reported by Bergenstal et al.1).
Baseline predictors of A1C reduction
Baseline A1C in both study groups, age at randomization, and age at diagnosis of diabetes in the SAP group and mean sensor glucose value in the MDI group predicted ≥0.5% A1C reduction without severe hypoglycemia (all P < 0.05; Table 1).
Table 1.
Predictors of Response to Treatment: P Value
| |
P value |
|||
|---|---|---|---|---|
| Baseline | SAP Model 1 | MDI Model 1 | SAP Model 2 | MDI Model 2 |
| A1C | 0.008 | 0.003 | 0.002 | 0.02 |
| Documented SMBG/day | NA | NA | 0.96 | 0.68 |
| BMI | NA | NA | 0.80 | 0.39 |
| Age at randomization | 0.01 | NA | 0.003 | 0.24 |
| Duration of diabetes | NA | NA | 0.37 | 0.33 |
| Age at diagnosis | NA | NA | 0.048 | 0.86 |
| Smoking (yes/no) | NA | NA | 0.87 | 0.44 |
| Alcohol use (yes/no) | NA | NA | 0.69 | 0.58 |
| Sex (M/F) | NA | NA | 0.50 | 0.60 |
| SD during 1-week screening | NA | NA | 0.96 | 0.22 |
| Mean sensor glucose value | NA | 0.01 | 0.88 | 0.10 |
Model 1 is a multivariate logistic regression model using backward selection keeping those variables with P < 0.05 that detects whether the characteristic predicts a −0.5% change in A1C without severe hypoglycemia. Model 2 is a univariate logistic regression model that detects whether the individual characteristic predicts a −0.5% change in A1C without severe hypoglycemia. P values represent correlation between baseline characteristic and 1 year −0.5% change in A1C without severe hypoglycemia.
BMI, body mass index; MDI, multiple daily injection; NA, not available (dropped from logistic backward selection procedure); SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
In a comparison of baseline characteristic values to mean 1-year A1C and mean A1C change (Table 2), higher baseline A1C predicted higher end point A1C and greater A1C reduction with SAP than MDI. Younger age at randomization (≤35 years) and diagnosis (≤16 years) was associated with slightly higher 1-year A1C and less differential change in A1C with SAP compared with MDI.
Table 2.
Baseline Characteristics Compared with 1-Year A1C
| |
No. of subjects |
Mean 1-year A1C [% (SD)] |
Mean change in A1C from baseline to 1 year [% (SD)] |
|||
|---|---|---|---|---|---|---|
| Baseline characteristic | SAP | MDI | SAP | MDI | SAP | MDI |
| A1C | ||||||
| ≤8 % | 65 | 60 | 7.1 (0.5) | 7.6 (0.7) | −0.7 (0.5) | −0.1 (0.7) |
| 8.1–9% | 85 | 88 | 7.4 (0.7) | 8.0 (0.8) | −1.1 (0.8) | −0.6 (0.8) |
| ≥9.1% | 16 | 15 | 7.7 (0.7) | 9.0 (1.1) | −1.6 (0.7) | −0.3 (1.0) |
| Age at randomization | ||||||
| ≤35 years | 59 | 58 | 7.4 (0.7) | 8.0 (1.0) | −0.7 (0.8) | −0.4 (0.9) |
| 36 – 50 years | 58 | 68 | 7.2 (0.7) | 8.0 (0.9) | −1.1 (0.7) | −0.3 (0.8) |
| ≥51 years | 49 | 37 | 7.3 (0.6) | 7.8 (0.6) | −1.1 (0.7) | −0.4 (0.7) |
| Age at diagnosis | ||||||
| ≤16 years | 61 | 70 | 7.3 (0.7) | 7.8 (0.8) | −0.8 (0.8) | −0.5 (0.7) |
| 17 – 30 years | 57 | 49 | 7.3 (0.6) | 8.0 (1.0) | −1.0 (0.7) | −0.2 (0.9) |
| 31 years | 48 | 44 | 7.3 (0.7) | 7.9 (0.9) | −1.1 (0.7) | −0.3 (0.8) |
| Mean sensor glucose value | ||||||
| ≤160 mg/dL | 50 | 48 | 7.2 (0.6) | 7.7 (0.7) | −1.0 (0.7) | −0.5 (0.7) |
| 161 – 190 mg/dL | 57 | 68 | 7.3 (0.7) | 7.9 (0.9) | −1.0 (0.7) | −0.3 (0.8) |
| ≥191 mg/dL | 59 | 47 | 7.4 (0.7) | 8.1 (0.9) | −1.0 (0.8) | −0.4 (0.9) |
MDI, multiple daily injection; SAP, sensor-augmented pump.
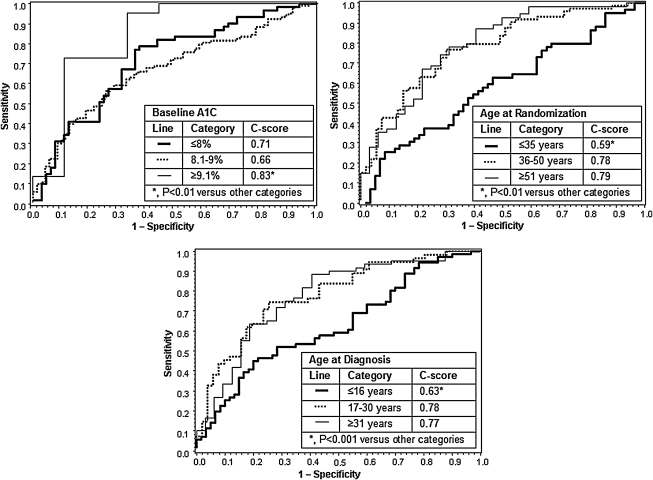
ROC curves (Fig. 1) showed that A1C ≥9.1%, age at randomization of ≥36 years, and age at diagnosis of ≥17 years were associated with higher C-scores. C-scores for categories of these characteristics were significantly different from one another (all P < 0.01). Similar results were found in an analysis of the treatment difference between SAP and MDI within baseline characteristic categories (Table 3).
FIG. 1.
Receiver operator characteristic curves of baseline A1C, age at randomization, and age at diagnosis plotted against 1-year ≥0.5% A1C reduction without severe hypoglycemia and adjusted for baseline A1C and mean sensor glucose values.
Table 3.
Treatment Difference Between Sensor-Augmented Pump and Multiple Daily Injection Therapy
| Variable | Treatment difference (% A1C)a | 95% CI (% A1C) | P value |
|---|---|---|---|
| Age at randomization | |||
| ≤35 years | −0.5 | −0.721, −0.192 | < 0.001 |
| 36–50 years | −0.8 | −1.005, −0.499 | < 0.001 |
| ≥51 years | −0.6 | −0.882, −0.265 | < 0.001 |
| Age at diagnosis | |||
| ≤16 years | −0.4 | −0.666, −0.164 | 0.001 |
| 17–30 years | −0.7 | −1.014, −0.462 | < 0.001 |
| ≥31 years | −0.7 | −1.034, −0.440 | < 0.001 |
| Baseline A1C | |||
| ≤8% | −0.5 | −0.792, −0.290 | < 0.001 |
| 8.1–9% | −0.5 | −0.750, −0.323 | < 0.001 |
| ≥9.1% | −1.3 | −1.762, −0.750 | < 0.001 |
Treatment differences are in favor of sensor-augmented pump therapy.
CI, confidence interval.
Conclusions
We are not aware of a device trial in which A1C lowering versus an active comparator was as great in type 1 diabetes as SAP therapy in STAR 3,1 but the necessary time and resource investment to initiate SAP therapy is high. We used statistical models to determine whether certain baseline characteristics were predictive of 1-year A1C benefit. We then used cutpoint comparisons with ROC curves to determine the relative benefit of SAP to MDI for subjects in different baseline categories. Higher baseline A1C in both study groups and older age at randomization and diagnosis in the SAP group were associated with greater benefit. Baseline CGM-derived values did not predict response to SAP or continued MDI therapy.
Although higher baseline A1C predicted a higher 1-year A1C in both study groups, the reduction in A1C at higher baseline values was greater for SAP than MDI. Specifically, the difference in 1-year A1C change for SAP subjects was 1.3% at A1C ≥9.1% where ongoing MDI showed very little benefit. However, the benefit at lower A1C (≤8%) was robust at 0.6%. Age at diagnosis of ≤16 years and age at randomization of ≤35 years were associated with similar benefits of SAP and MDI, approximately 0.3% each. ROC and treatment difference by baseline characteristic analyses, similarly, demonstrated greater 1-year A1C benefit of SAP among subjects with baseline A1C of ≥9.1%, age at randomization of ≥36 years, and age at diagnosis of ≥17 years.
These results are consistent with a recent study of CGM versus self-monitoring of blood glucose5 and several meta-analyses of insulin pump use versus MDI6–8 that have suggested associations between A1C reduction and baseline A1C or age, although not to the level of detail presented in our report. CGM was associated with greater A1C benefit among people with baseline A1C ≥8.0% and age ≥25 years.5 Insulin pump use was associated with greater A1C benefit among people with baseline A1C >6.5% to 10%4 and an age of ≥18 years.7 There were also statistical associations without specification of direction between A1C benefit from insulin pump use and baseline A1C and age; in that meta-analysis, however, lower severe hypoglycemia from insulin pump use was specifically associated with older age and higher baseline A1C.8
It is important to note that this report does not consider any post-baseline behaviors that may impact A1C benefit, such as the frequency of blood glucose self-monitoring or the number of boluses administered. As previously noted among the SAP group, increasing sensor wear was significantly associated with larger decrements in A1C.1 Limitations of the STAR 3 study include the fact that it was a necessarily unblinded comparison of SAP and MDI treatments, that it was not designed to evaluate the incremental effects of insulin pump therapy or CGM sensors in isolation, and that the A1C inclusion criteria (7.4–9.5%) may limit the generalizability of its results.
This report suggests that SAP, which is effective in lowering A1C in adults with type 1 diabetes inadequately controlled on MDI,1 has greater A1C-lowering benefit among adults with higher baseline A1C, older age at randomization, and older age at diagnosis (treatment difference ranged from −0.5% to −1.3%) than other adults in the study (treatment difference ranged from −0.4% to −0.5%).
Appendix
STAR 3 Study Group
The following clinical centers are listed in alphabetical order by city name. Primary investigators (PI), sub-primary investigators (Sub-PI), study coordinators (CO), and diabetes educators (DE) are listed with their affiliated centers:
Mountain Diabetes and Endocrine Center, PLLC, Asheville, NC: Wendy S. Lane, M.D. (PI); Kristen Arey (CO); Terri Przestrzelski, R.N., C.D.E. (DE)
Joslin Diabetes Center, Boston, MA: Sanjeev Mehta, M.D. (PI); Lori Laffel, M.D., M.P.H. (PI); Jyoti Aggarwal, M.H.S. (CO); Katherine Pratt, R.N., C.D.E. (DE)
University of North Carolina School of Medicine, Chapel Hill, NC: John B. Buse, M.D., Ph.D. (PI); Michelle Duclos, M.P.H., CCRC (CO); Joseph Largay, P.A.-C., C.D.E. (DE)
Ohio State University College of Medicine, Columbus, OH: Kwame Osei, M.D., FACE, FACP (PI); Cecelia Casey-Boyer, R.N., M.S., C.D.E. (CO, DE); Hollie Breedlove, M.S., R.D., L.D. (CO, DE)
University of Colorado Denver, Barbara Davis Center, Denver, CO: Robert Slover II, M.D. (PI); Stephanie Kassels, R.N., B.S.N. (CO, DE); Sally Sullivan, R.N., C.D.E. (DE)
Duke University Medical Center, Diabetes Research Clinic, Durham, NC: Jennifer B. Green, M.D. (PI); Jennifer English-Jones, R.N., C.D.E. (CO, DE)
DeVos Children's Hospital, Grand Rapids, MI: Michael A. Wood, M.D. (PI); Emily Gleason, R.N., M.S.N. (CO); Laura Wagner, R.N. (DE), Thomas Symington, R.N. (CO), Lora Kleis, R.N. (DE)
East Carolina University, Diabetes and Obesity Center, Greenville, NC: Robert J. Tanenberg, M.D., FACP (PI); Carolyn Knuckey, L.P.N. (CO, DE); Savanna Cummings, R.D., C.D.E. (DE)
Rocky Mountain Diabetes and Osteoporosis Center, Idaho Falls, ID: David R. Liljenquist, M.D. (PI); John E. Liljenquist, M.D. (PI); Becky Sulik, R.D., L.D., C.D.E. (CO, DE)
Kingston General Hospital, Kingston, ON, Canada: Robyn L. Houlden, M.D., FRCPC (PI); Trish LaVallee, R.N. (CO, DE); Adrianna Breen, R.N., B.S.N. (DE); Ruth Barrett, R.N., C.D.E. (DE)
Scripps Institute, La Jolla, CA: George Dailey, M.D. (PI); Athena Philis-Tsimikas, M.D. (Sub-PI); JoAnn Shartel, R.N., C.D.E. (DE)
Kentucky Diabetes Endocrinology Center, Lexington, KY: Lyle Myers, M.D. (PI); Diane Ballard, R.N., B.S.N., C.D.E., C.P.T. (CO, DE)
Endocrinology Diabetes Clinic, University of Wisconsin Health, Madison, WI: Melissa Meredith, M.D. (PI); Connie Trantow, M.S., CCRC (CO, DE)
Diabetes Research Institute, Miami, FL: Luigi F. Meneghini, M.D., M.B.A. (PI); Jane Sparrow-Bodenmiller, R.N., C.D.E. (CO); Robert Agramonte (DE)
Minnesota International Diabetes Center, Minneapolis, MN: Richard M. Bergenstal, M.D. (PI); Amy B. Criego, M.D. (Sub-PI); Sarah Borgman, B.S.N., R.N., C.D.E. (CO, DE)
Vanderbilt University, Nashville, TN: Michael E. May, M.D., Ph.D., C.D.E. (PI); Stephen N. Davis, M.D., MBBS, FRCP, FACP (PI); Connie Root, R.N., M.S.N. (CO, DE)
Yale University, New Haven, CT: Stuart A. Weinzimer, M.D., FAAP (PI); William Tamborlane, M.D., FAAP, FACE (Sub-PI), Lori Carria, M.S. (CO); Jennifer Sherr, M.D. (DE)
Children's Hospital of Orange County, Orange, CA: Mark Daniels, M.D. (PI); Jody S. Krantz, M.D. (PI); Susan Clark, M.D. (Sub-PI); Debbie Warner, R.N., C.D.E. (DE); Heather Speer, M.P.H., CCRC, CHES, C.D.E. (CO); Joane Less, R.N., B.S.N., M.B.A. (CO)
Children's Hospital of Philadelphia, Philadelphia, PA: Steve M. Willi, M.D. (PI); Tammy Calvano, M.A. (CO, DE); Erin Garth, R.N., M.S.N. (CO, DE)
Oregon Health and Science University, Portland, OR: Andrew A. Ahmann, M.D. (PI); Christopher Bogan (CO); Bethany Wollam (CO)
Mayo Clinic, Rochester, MN: Yogish C. Kudva, M.D. (PI); Betty Wirt, L.P.N. (DE); Arlene Morris (CO)
University of Rochester School of Medicine and Dentistry, Rochester, NY: Craig Orlowski, M.D. (PI); Sue Bates, R.N., M.S.N. (CO, DE); Barbara Johnson
Endocrine Research Solutions, Inc, Roswell, GA: John C. Reed III, M.D., C.D.E. (PI); Jessica Tapia (CO); Karla Wardell (CO, DE); Stacey Newsome, C.D.E. (DE)
Memorial University of Newfoundland, Health Science Center, St. John's, NL, Canada: Carol Joyce, M.D. (PI); Daisy Gibbons, R.N. (CO); Juanita O'Leary, R.N., C.D.E. (DE)
Washington University in St. Louis School of Medicine, St. Louis, MO; Neil H. White, M.D., C.D.E. (PI); Melanee Coleman, R.N. (CO, DE)
Children's Hospital of St. Paul, St. Paul, MN: Robert C. McEvoy, M.D. (PI); Laura Gandrud, M.D. (PI); Catherine Girard, M.S. (CO, DE); Jayne Chatterton, M.A., C.N.P., C.D.E. (DE)
Utah Diabetes Center, Salt Lake City, UT: Carol M. Foster, M.D. (PI); Trina Brown, APRN, M.S.N. (CO, DE); Elizabeth Nuttall (CO, DE)
Toronto General Hospital, Toronto, ON, Canada: Bruce A. Perkins, M.D., MPH, FRCP (PI); Andrej Orszag (CO, DE)
Endocrine Research, Inc., Vancouver, BC, Canada: Hugh Tildesley, M.D., FRCPC (PI); Betty Pottinger, R.N., C.D.E. (CO, DE)
Mid-America Diabetes Associates, PA, Wichita, KS: Richard A. Guthrie, M.D., FAAP, FACE (PI); Phillip Challans, M.D. (Sub-PI); Julie Dvorak, R.N., B.S.N., C.D.E. (CO); Belinda Childs, ARNP, M.S.N., C.D.E., BC-ADM, R.N. (DE)
Steering Committee
Scott Lee, M.D. (Chair and Medical Director): Medtronic, Inc, Northridge, CA
Tadej Battelino, M.D., Ph.D.: University Children's Hospital, Ljubljana, Slovenia
Stephen N. Davis, M.D., MBBS, FRCP, FACP: University of Maryland Medical Center, Baltimore, MD
Edward S. Horton, M.D.: Joslin Diabetes Center, Boston, MA
Richard R. Rubin, Ph.D., C.D.E.: Johns Hopkins University, Baltimore, MD
Kevin A. Schulman, M.D., M.B.A.: Duke University, Durham, NC
William V. Tamborlane, M.D., FAAP, FACE: Yale University, New Haven, CT
Acknowledgments
This study was supported by Medtronic; Novo Nordisk, which supplied all insulin aspart used in the study; and LifeScan, Bayer Healthcare, and Becton Dickinson, which supplied blood glucose meters used in the study.
Author Disclosure Statement
J.B.B., G.D., A.A.A., R.M.B., J.B.G., and R.J.T. were primary investigators in STAR 3, for which their institutions received funding. J.B.B. reports that for his services the University of North Carolina School of Medicine has received consulting fees from Novo Nordisk, Amylin, Becton Dickinson, Eli Lilly, Hoffmann–La Roche, GlycoMark, Wyeth, Daiichi Sankyo, Bristol-Myers Squibb, Bayhill Therapeutics, LipoScience, MannKind, Valeritas, MicroIslet, GlaxoSmithKline, Abbott, Exsulin, and GI Dynamics; funding from Novo Nordisk for expert testimony; grant support from Amylin, Novo Nordisk, Medtronic, Lilly, Novartis, Tolerex, Osiris, Halozyme, Pfizer, Hoffmann–La Roche, Interkrin, Merck, sanofi-aventis, DexCom, Johnson & Johnson, Bristol-Myers Squibb, Fujisawa, and Novartis; and travel and accommodation reimbursement from Novo Nordisk, Amylin, Medtronic, Novartis, Tolerex, Becton Dickinson, Eli Lilly, Hoffmann–La Roche, Fujisawa, GlycoMark, Wyeth, Daiichi Sankyo, Bristol-Myers Squibb, Bayhill Therapeutics, Osiris, Interkrin, Merck, sanofi-aventis, DexCom, Johnson & Johnson, Halozyme, Pfizer, LipoScience, MannKind, Valeritas, MicroIslet, GlaxoSmithKline, Abbott, Exsulin, and GI Dynamics. He also reports having an equity interest in Insulet. G.D. reports receiving consulting fees from sanofi-aventis and Merck, travel reimbursement from Medtronic, grant support from Medtronic, and honoraria from sanofi-aventis and Merck. A.A.A. reports that he has received fees for board membership from Novo Nordisk and honoraria from Amylin, Eli Lilly, Animas, the National Diabetes Education Initiative, and Merck; he also reports that Oregon Health and Science University has received grant support from Amylin, Medtronic, Novo Nordisk, and Eli Lilly. R.M.B. reports that the International Diabetes Center has received consulting fees for his services from Abbott Diabetes Care, Amylin, Bayer, Calibra, Eli Lilly, Intarcia, MannKind, Medtronic, Novo Nordisk, Pfizer, ResMed, Roche, sanofi-aventis, and Takeda; grant support from Abbott Diabetes Care, Biodel, Eli Lilly, Hygieia, Intuity, LifeScan, Medtronic, Novo Nordisk, Pfizer, ResMed, Roche, sanofi-aventis, Takeda, UnitedHealth Group, and Valeritas; honoraria from Abbott Diabetes Care, Amylin, Bayer, Calibra, Eli Lilly, Intarcia, MannKind, Medtronic, Novo Nordisk, Pfizer, ResMed, Roche, sanofi-aventis, and Takeda; royalties on his behalf for the Betty Crocker Diabetes Cookbook; and travel and accommodation reimbursements from Abbott Diabetes Care, Amylin, Bayer, Biodel, Calibra, Eli Lilly, Hygieia, Intarcia, Intuity, LifeScan, MannKind, Medtronic, Novo Nordisk, Pfizer, ResMed, Roche, sanofi-aventis, Takeda, UnitedHealth Group, and Valeritas. He also reports stock ownership in Merck through a family inheritance. J.B.G. reports that she has received consulting fees from VeroScience and honoraria from Takeda Pharmaceuticals and Merck; she also reports that Duke University has received grant support from Medtronic, Merck, and Amylin for her services. R.J.T. reports that East Carolina University has received grant support from Medtronic and sanofi-aventis, Johnson and Johnson, and CPEX Pharmaceuticals; he also reports that he is on the advisory board for sanofi-aventis and the speaker's bureau for sanofi-aventis, Takeda Pharmaceuticals, and Pfizer. T.P. reports full-time employment by and stock ownership in Medtronic and receiving compensation for manuscript preparation. Q.Y. reports full-time employment by Medtronic and receiving compensation for biostatistical analysis. All authors participated in manuscript writing and revision. J.B.B., G.D., A.A.A., R.M.B., J.B.G., and R.J.T. were principal investigators in STAR 3. Data were analyzed at Medtronic by Q.Y. R.M.B. and J.B.G. researched data, contributed to discussion, and reviewed/edited manuscript. R.J.T. contributed to discussion and reviewed/edited the manuscript.
References
- 1.Bergenstal RM. Tamborlane WV. Ahmann A. Buse JB. Dailey G. Davis SN. Joyce C. Peoples T. Perkins BA. Welsh JB. Willi SM. Wood MA. STAR 3 Study Group: Effectiveness of sensor-augmented insulin pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 2.Davis SN. Horton ES. Battelino T. Rubin RR. Schulman KA. Tamborlane WV. STAR 3 randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Diabetes Technol Ther. 2010;12:249–255. doi: 10.1089/dia.2009.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green JB. Ahmann A. Bergenstal RM. Dailey G. Tanenberg R. Buse JB. STAR 3 Study Group: Glucose control in adults during a 1-year randomised controlled trial comparing sensor-augmented pump therapy and multiple daily injection therapy: STAR 3 study [abstract] Diabetologia. 2010;53(Suppl 1):S24. [Google Scholar]
- 4.Ahmann A. Buse JB. Bergenstal RM. Tanenberg R. HbA1c and sensor use in adults during a 1-year randomised controlled trial comparing sensor-augmented pump therapy and multiple daily injection therapy [abstract] Diabetologia. 2010;53(Suppl 1):S401. [Google Scholar]
- 5.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32:1947–1953. doi: 10.2337/dc09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retnakaran R. Hochman J. DeVries JH. Hanaire-Broutin H. Heine RJ. Melki V. Zinman B. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care. 2004;27:2590–2596. doi: 10.2337/diacare.27.11.2590. [DOI] [PubMed] [Google Scholar]
- 7.Misso ML. Egberts KJ. Page M. O'Connor D. Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;1:CD005103. doi: 10.1002/14651858.CD005103.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Pickup JC. Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765–774. doi: 10.1111/j.1464-5491.2008.02486.x. [DOI] [PubMed] [Google Scholar]