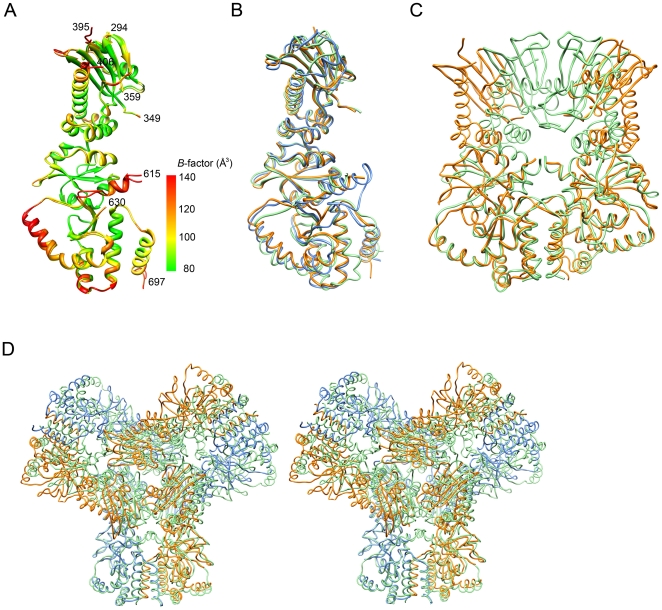
Figure 3. Comparison of HSP90 structures.
(A) Disordered regions of MC-HSP90. The protomers A and B in the C2221 crystal are superimposed with RMSD of 0.637 Å between 367 Cα atom pairs. The residue number of N- and C-termini and the terminal ends of disordered loops in protomer A are indicated. The protein models are presented with ramped colors according to the B values. The start of the curved α-helix (α9) and the extended arm (α10) at C domain show high temperature factors. (B) The monomer structure of human MC-HSP90 (orange) is superimposed on the MC domains of closed-form yeast HSP82 (green) and open-form canine GRP94 (blue). (C) The dimer structure of human MC-HSP90 (orange) is superimposed on the MC domains of close-form yeast HSP82 (green). (D) Stereo view of superposed hexameric structures. The human MC-HSP90 hexamer and the N-terminal truncated yeast HSP82 (PDB: 2CGE) hexamer are superimposed. The protomers of Human MC-HSP90 dimer are colored in blue and orange, respectively, and six protomers of yeast HSP82 are colored in green. See Video S1 for more comprehensive aspects.